- Business Process Management (BPM)Document Management System (DMS)Electronic Quality Management System (QMS)Risk, Governance & Compliance (GRC)Low Code Rapid Application Development (LC)Business Continuity Management (BCM)Enterprise Architecture (EA)Business Process Management (BPM)Document Management System (DMS)
- Document Control Overview
- AI Content Creation & Improvement
- Policy & Procedure Management (SOP)
- AI Content Mining Parser
- Collaboration & Governance
- Data Migration & Integration
- Interfacing Offline App
 Electronic Quality Management System (QMS)
Electronic Quality Management System (QMS)- Quality Management System Overview
- Document Control & Records Management
- Audit & Accreditation Management
- Corrective & Preventative Action
- Quality Event (Non-conformity / Complaint/ Compliance)
- Risk Management
- Incident Management
- Environmental Health & Safety
- Product & Supplier Management (SCAR)
- Training Management
- Control Management
- Action Items Management
- Management Review
- FMEA
- Pharmacovigilance
- Data Migration & Integration
 Risk, Governance & Compliance (GRC)
Risk, Governance & Compliance (GRC)- Risk, Governance & Compliance Overview
- Risk & Control Management
- Regulatory Compliance
- Collaboration & Governance
- Data Migration & Integration
- Interfacing Offline App
 Low Code Rapid Application Development (LC)
Low Code Rapid Application Development (LC)- Low Code Automation Platform Overview
- Electronic Web Form Design (eFORMS)
- Database Table Entity Designer
- System Integration Designer
- Design & Manage Tasks
- Design & Manage BPMS Apps
- Custom Rules/Guards/Actions
- Electronic Services
- User Homepage
- BAM (Business Activity Monitoring)
- Custom Dashboard Design
- Data Migration & Integration
 Business Continuity Management (BCM)
Business Continuity Management (BCM)- Business Continuity Management Overview
- Business Impact Analysis
- Disaster Recovery Simulation
- Action Item Management
- Mass Notification Management
- Asset Management
- Interfacing Offline App
 Enterprise Architecture (EA)
Enterprise Architecture (EA) - IndustriesRegulatory ComplianceUse CasesLearning CenterFramework & PracticesIndustries
- Healthcare
- Medical Device Technology
- Life Science, Pharmaceutical
- Aerospace & Defense
- Airlines and Aviation
- Media & Telecommunications
- Government and Military
- Technology
- Energy
- Logistics & Port Operations
- Banking & Capital Markets
- Retail & Consumer
- Consulting
- Education
- Engineering & Construction
- Manufacturing
- Financial Services
- Insurance
- Chemicals
Regulatory Compliance- Regulatory Compliance
- ISO
- ISO 9001 (guide)
- ISO 9001:2026 (preparation)
- ISO 17025
- ISO 27000
- ISO 27001
- ISO27002
- ISO 42001
- EU AI Act
- SOC 2 Type 1 & 2
- Sarbanes Oxley
- GxP
- GRC
- Basel
- Digital Signature
- GDPR
- IFRS
- NIST SP 800-53
 Use Cases
Use Cases- Quality Management System (QMS)
- Digital Transformation
- Continuous Improvement
- Governance, Risk & Compliance
- Knowledge Management
- System Deployment (ERP, CRM…)
 Learning CenterFramework & Practices
Learning CenterFramework & Practices - AboutCustomer SuccessPartners

What is ALCOA+
Please Select contact form.
Learn how ALCOA+ principles safeguard data quality, protect patients, and ensure regulatory success across life sciences and beyond.

What is ALCOA+?
ALCOA+ is a set of principles that define how data should be created, stored, and maintained to ensure integrity throughout its lifecycle. Originating from regulatory bodies such as the FDA and EMA, ALCOA stands for Attributable, Legible, Contemporaneous, Original, and Accurate—with the “+” adding critical extensions: Complete, Consistent, Enduring, and Available.
These principles are foundational to Good Documentation Practices (GDP) and Good Manufacturing Practices (GMP) and have become central to digital compliance in highly regulated sectors. Violations—such as data fabrication or retrospective edits—can lead to rejected submissions, costly rework, or even product recalls.
In today’s digital era, where electronic systems replace paper records, ALCOA+ ensures that integrity is not lost in convenience. AI-enhanced systems like Interfacing’s EPC help maintain compliance by logging activities automatically, detecting anomalies, and managing approval chains in a traceable, auditable format.
History and Current Impact
The concept of ALCOA dates back to the 1990s when the FDA sought to formalize expectations around lab data. It gained momentum with the rise of electronic record-keeping and was later expanded by EMA and PIC/S to ALCOA+—emphasizing the role of enduring and accessible data across its lifecycle.
Today, ALCOA+ is a benchmark in global regulatory inspections across pharma, biotech, medical devices, and increasingly in sectors like food safety, environmental testing, and clinical research. It's no longer a best practice—it’s a mandate.


Why ALCOA+ Compliance is Needed
ALCOA+ is critical for ensuring that data used in regulatory submissions, audits, or public health decisions is credible and reproducible. Non-compliance can delay product approvals, damage reputations, or result in enforcement actions. ALCOA+ brings accountability, visibility, and consistency to digital records—whether you're running a clinical trial or managing a production facility.


Real-World Use Case
Background:
A global biosimilar manufacturer preparing a biologics license application (BLA) faced multiple ALCOA+ violations during a mock audit. Issues included inconsistent lab record entries, lack of version control, and unauthorized file access.
Response:
The quality team partnered with Interfacing to digitize all laboratory and batch records using EPC. Each record was tagged with unique IDs, source ownership, and time-of-entry logs. AI flagged historical entries lacking metadata and offered correction workflows with justification fields.
Transformation with Interfacing:
EPC automated the approval chain for lab results and batch reviews, embedded SOPs into data entry templates, and enforced review timelines via AI-generated alerts. The team used dashboards to track record aging, missing fields, and audit preparedness.
Outcome:
In under four months, the organization closed over 300 identified compliance gaps, passed a regulatory pre-inspection with zero major findings, and received full submission approval. EPC continues to be their central data integrity platform across labs and production.
Relevant Industries
ALCOA+ applies anywhere critical decisions are made based on data. However, adoption is most prevalent where regulatory oversight is intense and the consequences of data tampering are severe.
Life Sciences
Pharmaceutical and biotech companies must prove that their data—from clinical trials to production batches—is reliable and tamper-proof. ALCOA+ compliance is routinely reviewed during FDA, EMA, and Health Canada inspections. Interfacing’s EPC platform digitizes lab notebooks, audit trails, and approval chains with AI-driven alerts when metadata anomalies are detected.
Medical Devices
Under frameworks like ISO 13485 and 21 CFR Part 820, medical device manufacturers are expected to maintain traceable design, testing, and post-market data. EPC links documents and records to processes, people, and controls, ensuring compliance with both ALCOA+ and QMS regulations.
Contract Manufacturing & CROs
CMOs and CROs often work with multiple sponsors, each with unique requirements. ALCOA+ ensures that third-party-generated data maintains the same level of control and traceability. Interfacing helps standardize digital record practices across client projects with version-controlled repositories and role-based access.
Food and Environmental Testing
Data integrity isn’t just a pharma concern. Labs testing food, water, and environmental samples are increasingly required to adhere to ALCOA+ to meet ISO 17025 standards. With EPC, lab records can be digitized, locked, and linked to chain-of-custody protocols for defensible data.
Steps to ALCOA+ Compliance
Perform a Data Integrity Gap Assessment
Evaluate your systems, processes, and documentation practices against ALCOA+ principles. Interfacing’s EPC uses AI to scan digital workflows for missing metadata, unverifiable timestamps, or orphaned records.
Map Roles and Responsibilities
Assign ownership of data entry, review, and archiving. EPC’s org chart integration and workflows ensure responsibilities are mapped and validated.
Digitize and Control Documentation
Implement a system that version-controls records, restricts access, and logs changes. EPC automates record lifecycle management, ensuring traceability and audit readiness.
Train and Audit
Use EPC’s embedded training modules and audit dashboards to raise awareness of ALCOA+ across departments and verify ongoing adherence.
Enable Continuous Monitoring
Let AI monitor anomalies in real-time—flagging irregular access, late entries, or missing approvals before they become audit findings.

Common Pitfalls to Avoid
Backdating Entries
A leading cause of regulatory non-compliance. EPC’s timestamp features prevent undetected retroactive edits.
Paper-Digital Hybrids
Mixing paper and digital records without clear SOPs leads to incomplete data chains. Digitize your workflows fully with EPC.
Unassigned Ownership
Without clear accountability, data integrity efforts fail. EPC embeds ownership directly into process maps and approval flows.
Static Systems
Compliance isn’t a one-time task. EPC allows continuous adaptation to changes in regulation, user roles, and record types.
How AI Can Help with ISO 14001
AI makes ISO 14001 more than manageable—it makes it predictive. From real-time emissions tracking to anomaly detection in water or energy usage, Interfacing’s AI-enhanced tools help anticipate risks, streamline data collection, and support decision-making.
With machine learning models embedded into EPC, users can forecast the impact of process changes on compliance targets, detect reporting inconsistencies, and automate audit readiness.
How Interfacing Helps
Interfacing’s EPC platform supports ALCOA+ compliance by digitizing and centralizing all data entry, documentation, and audit processes. With integrated AI, EPC can proactively flag integrity risks, enforce control ownership, and keep your data lifecycle compliant and audit-ready.
Whether you’re in life sciences, manufacturing, or regulated testing, EPC aligns every data touchpoint to ALCOA+—with zero guesswork and full traceability.

Ensure Process & Quality Governance
Interfacing’s Enterprise Process Center® (EPC) allows you to define, document, and enforce ISO 9001 quality controls organization-wide. Policies, procedures, and SOPs are directly tied to processes and roles—ensuring your QMS is embedded in everyday operations with full transparency. .

Eliminate Manual Errors with AI-Driven QMS
Standardizing documentation and workflows within EPC removes the need for spreadsheets and disconnected systems. Our AI flags outdated procedures, suggests process improvements, and helps maintain version control—reducing the risk of non-compliance and audit issues.

Gain Full Audit Readiness & Traceability
EPC provides real-time traceability across all quality processes—from document approvals to CAPA workflows. With automated audit trails and visual process maps, you’re always ready for certification audits and internal reviews, no last-minute scrambling required

Improve Operational Efficiency Without Sacrificing Compliance
Interfacing’s QMS automates routine tasks such as change control, training sign-offs, and document updates. This not only frees up valuable staff time, but also ensures consistent adherence to ISO 9001 guidelines across all departments and geographies.

Reduce the Cost of Quality Compliance
With centralized process governance and AI-enhanced workflows, EPC reduces the overhead of maintaining your QMS. From smarter audit prep to fewer non-conformities, organizations save time, cut errors, and accelerate their path to ISO 9001 certification.

Build a Culture of Continuous Improvement
ISO 9001 is about more than passing audits—it’s about evolving your organization. Interfacing’s platform helps identify quality gaps, track corrective actions, and promote ongoing learning and accountability—turning compliance into a competitive advantage.
Why Choose Interfacing?
With over two decades of AI, Quality, Process, and Compliance software expertise, Interfacing continues to be a leader in the industry. To-date, it has served over 500+ world-class enterprises and management consulting firms from all industries and sectors. We continue to provide digital, cloud & AI solutions that enable organizations to enhance, control and streamline their processes while easing the burden of regulatory compliance and quality management programs.
To explore further or discuss how Interfacing can assist your organization, please complete the form below.

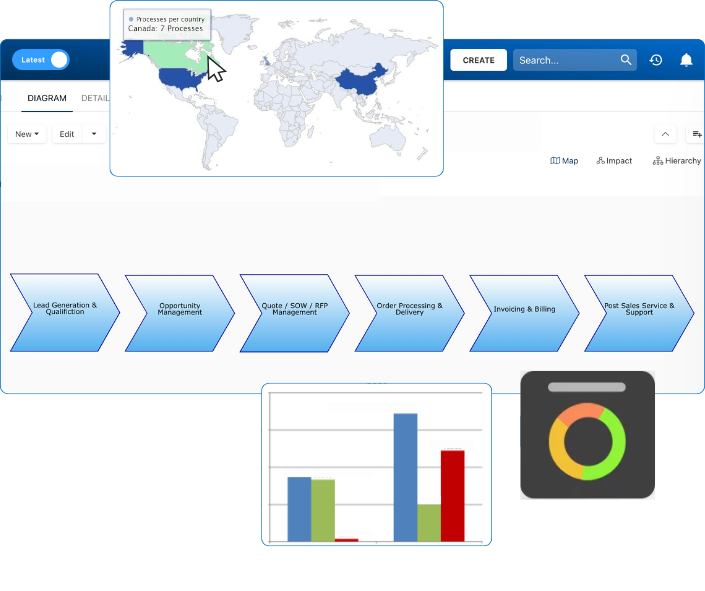
Documentation: Driving Transformation, Governance and Control
• Gain real-time, comprehensive insights into your operations.
• Improve governance, efficiency, and compliance.
• Ensure seamless alignment with regulatory standards.

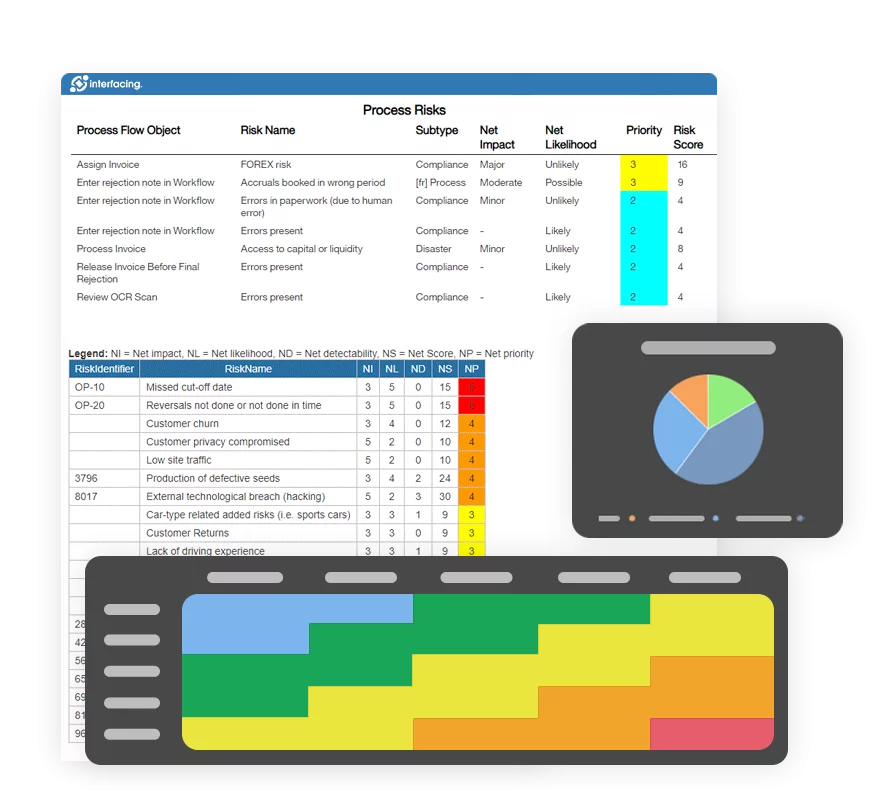
eQMS: Automating Quality & Compliance Workflows & Reporting
• Simplify quality management with automated workflows and monitoring.
• Streamline CAPA, supplier audits, training and related workflows.
• Turn documentation into actionable insights for Quality 4.0

Low-Code Rapid Application Development: Accelerating Digital Transformation
• Build custom, scalable applications swiftly
• Reducing development time and cost
• Adapt faster and stay agile in the face of
evolving customer and business needs.
AI to Transform your Business!
The AI-powered tools are designed to streamline operations, enhance compliance, and drive sustainable growth. Check out how AI can:
• Respond to employee inquiries
• Transform videos into processes
• Assess regulatory impact & process improvements
• Generate forms, processes, risks, regulations, KPIs & more
• Parse regulatory standards into requirements

Request Free Demo
Document, analyze, improve, digitize and monitor your business processes, risks, regulatory requirements and performance indicators within Interfacing’s Digital Twin integrated management system the Enterprise Process Center®!
Trusted by Customers Worldwide!
More than 400+ world-class enterprises and management consulting firms