- Business Process Management (BPM)Document Management System (DMS)Electronic Quality Management System (QMS)Risk, Governance & Compliance (GRC)Low Code Rapid Application Development (LC)Business Continuity Management (BCM)Enterprise Architecture (EA)Business Process Management (BPM)Document Management System (DMS)
- Document Control Overview
- AI Content Creation & Improvement
- Policy & Procedure Management (SOP)
- AI Content Mining Parser
- Collaboration & Governance
- Data Migration & Integration
- Interfacing Offline App
 Electronic Quality Management System (QMS)
Electronic Quality Management System (QMS)- Quality Management System Overview
- Document Control & Records Management
- Audit & Accreditation Management
- Corrective & Preventative Action
- Quality Event (Non-conformity / Complaint/ Compliance)
- Risk Management
- Incident Management
- Environmental Health & Safety
- Product & Supplier Management (SCAR)
- Training Management
- Control Management
- Action Items Management
- Management Review
- FMEA
- Pharmacovigilance
- Data Migration & Integration
 Risk, Governance & Compliance (GRC)
Risk, Governance & Compliance (GRC)- Risk, Governance & Compliance Overview
- Risk & Control Management
- Regulatory Compliance
- Collaboration & Governance
- Data Migration & Integration
- Interfacing Offline App
 Low Code Rapid Application Development (LC)
Low Code Rapid Application Development (LC)- Low Code Automation Platform Overview
- Electronic Web Form Design (eFORMS)
- Database Table Entity Designer
- System Integration Designer
- Design & Manage Tasks
- Design & Manage BPMS Apps
- Custom Rules/Guards/Actions
- Electronic Services
- User Homepage
- BAM (Business Activity Monitoring)
- Custom Dashboard Design
- Data Migration & Integration
 Business Continuity Management (BCM)
Business Continuity Management (BCM)- Business Continuity Management Overview
- Business Impact Analysis
- Disaster Recovery Simulation
- Action Item Management
- Mass Notification Management
- Asset Management
- Interfacing Offline App
 Enterprise Architecture (EA)
Enterprise Architecture (EA) - IndustriesRegulatory ComplianceUse CasesLearning CenterFramework & PracticesIndustries
- Healthcare
- Medical Device Technology
- Life Science, Pharmaceutical
- Aerospace & Defense
- Airlines and Aviation
- Media & Telecommunications
- Government and Military
- Technology
- Energy
- Logistics & Port Operations
- Banking & Capital Markets
- Retail & Consumer
- Consulting
- Education
- Engineering & Construction
- Manufacturing
- Financial Services
- Insurance
- Chemicals
Regulatory Compliance- Regulatory Compliance
- ISO
- ISO 9001 (guide)
- ISO 9001:2026 (preparation)
- ISO 17025
- ISO 27000
- ISO 27001
- ISO27002
- ISO 42001
- EU AI Act
- SOC 2 Type 1 & 2
- Sarbanes Oxley
- GxP
- GRC
- Basel
- Digital Signature
- GDPR
- IFRS
- NIST SP 800-53
 Use Cases
Use Cases- Quality Management System (QMS)
- Digital Transformation
- Continuous Improvement
- Governance, Risk & Compliance
- Knowledge Management
- System Deployment (ERP, CRM…)
 Learning CenterFramework & Practices
Learning CenterFramework & Practices - AboutCustomer SuccessPartners

ISO – The International Organization for Standardization
Please Select contact form.
A simple approach.

ISO – The International Organization for Standardization
Driving ISO initiatives with Business Process Management (BPM) creates far-reaching value for your organization, going beyond the ISO audit certification. While ISO standards are extremely useful to organizations, some of the standards lack a comprehensive outlook of the needs of modern businesses. Beyond a standard designed to facilitate international trade, ISO certification provides value in the form of marketing appeal and by fulfilling customer requirements. In order to achieve optimal business practices that will maximize your ROI, industry best practices provide ISO’s missing components.
Understanding ISO: Definition
ISO Standards are documents which provide guidelines, requirements and specifications to ensure products, processes and services fit their purpose. Economic uncertainty has forced companies to find ways to become more efficient in order to maintain profitability and be globally competitive. Formal performance improvement programs like ISO 9000 help to improve quality and operational efficiency, granting your company a competitive edge.
ISO 9001
International standard that specifies requirements for a QMS. It is the most popular standard in the ISO 9000 series and the only standard in the series to which organizations can certify.
First published in 1987 by the International Organization for Standardization (ISO). The current version of ISO 9001 was released in September 2015.
ISO 13485
In short, ISO 13485 is an internationally recognized standard that the following countries have adopted: Europe, Canada, Australia and other markets. Excluding Canada, the application of ISO 13485 is not a requirement but is the de facto standard in use today as a measurement of full QMS compliance set forth on medical device regulations.
ISO 14000
Environmental Management ISO 14000 ensures that a company minimizes the effect its activities have on the environment by implementing specific controls at the process level. ISO 14000 enables companies to reduce the penalties and fines conferred when environmental laws are breached. Furthermore, the adoption of ISO 14000 reduces waste, cutting down overhead, and ensuring the efficient use of materials.
ISO 20000
Technology Management ISO 20000 is an IT governance initiative intended to standardize IT policy by adopting standard best-practice processes in IT service. IS0 20000 is quickly becoming integral to modern business, as IT and business become more and more reliant on each other. By attaining compliance under ISO 20000, your company can increase efficiency in its delivery of IT services by providing a solid technology framework.
ISO 27000
The ISO 27000 international series of standards is the leading measurement metric for Security techniques as they relate to Information Security Management Systems. It is composed of a combination of policies and processes for organizations to form a framework to protect their information through the adoption of an Information Security Management System (ISMS). ISO 27001 is the most referenced standard in this series for certification.
ISO 27001 Cloud-solution
As part of our ongoing commitment to compliance and ensuring that our clients meet their regulatory requirements, we are always on the lookout for ways to help our clients attain and maintain full compliance. Interfacing is ISO 27001 certified and we are partnering with Amazon Web Services (AWS) for cloud-hosting since their commitment to compliance is proven, with global data centers compliance to SOC 1 Type II and ISO 27001. For more information on AWS compliance for ISO 18345, FDA QSR and GxP, please refer to their compliance program.
Electronic Quality Management System (eQMS) & ISO
Interfacing is here to assist you with the always evolving changes to compliance requirements that need to be addressed immediately. We lead the way to corporate transparency with our easy to deploy and comprehensive digital Integrated Management System (IMS) solutions for all your compliance, risk and process management needs.

Customer Focus
IMS allows business users to define and share your organization’s governing policies, so as to ensure compliance. By automatically applying these policies to the related processes, IMS takes care of compliance for you.

Leadership
IMS standardizes processes to meet compliance with corporate and regulatory policies, eliminating many of the errors created by manual compliance solutions. By automating the processes and providing appropriate reporting and tracking, errors can be avoided, resulting in significant cost savings.

Engagement of People
Complete audit trails that allow you to track all processes and changes can be extracted and reported – a vital component for managing change and ensuring process compliance to standards and regulations. This improved visibility allows you to identify and correct weaknesses with ease.

Process Approach
IMS streamlines processes and their development and allows for their fully integrated end-to-end management. Our solution helps you cut through the clutter and maximize the effectiveness of your processes every time.

Improvement
IMS lets you streamline and automate your compliance initiatives, including reporting and executing change, which lowers the soft and hard costs of compliance and risk management.

Evidence Based Decision Making
One example is better control awareness on how gaps and faulty assumptions suddenly come to light through implementation of SOX compliance procedures. Inadequate controls are quickly identified as complex manual processes are automated through implementation of newer, more accurate workflows.

Relationship Management
IMS lets you streamline and automate your compliance initiatives, including reporting and executing change, which lowers the soft and hard costs of compliance and risk management.
Understanding ISO compliance with Enterprise Process Center® (EPC)
We understand that the requirements placed on organizations in terms of compliance are very high and that ISO 9000, ISO 13845, ISO 14000, ISO 20000, ISO 27000, is an essential part of that program. By using our customized solutions, your company gains the accountability and consistency that will give you a cutting edge over your competition. Our tools ensure full visibility from end-to-end, all the way from the creation and amendment of a regulation to the approval and revision of the content through to the update and retraining of employees for standard operating procedures (SOPs). We see the full lifecycle management as moving parts of a complete ecosystem and that’s why are unique approach that combines information security, regulatory requirements, documents, processes, work instructions, and governance.
Conduct Impact Analysis
Analyze your records for downstream impacts, and analyze the potential impacts on policies, SOPs, business units and related records.
Manage Content
Manage the individual pieces of information, assign owners, and ensure governance through approval cycles, and change requests.
Digital Signature
We fully support digital signature to ensure that the audit trail of all content is secure, time-stamped, with accurate and complete copies of records available for inspection throughout the retention period.
Ensure Transparency
Full visibility to understand where records are used and their applicability. You can also maintain digital content with clear accountability, including roles and responsibilities.
Approval and Governance Workflow
Integrated and embedded approval workflows to ensure strict control over the change of your records, including validation of changes, evaluation of impacts and highlighting changes.
Digital SOPs
Generate complete customizable output of processes and related records such as regulations in a ready-to-print and exportable Word format. You no longer need to manage SOP on paper! The digital SOP is in-sync all the time.
Encourage Collaboration
By uniting goals and creating a common framework for your teams, they will be able to cooperate strategically, create change requests, and assign tasks to implementers.
Why Choose Interfacing?
With over two decades of AI, Quality, Process, and Compliance software expertise, Interfacing continues to be a leader in the industry. To-date, it has served over 500+ world-class enterprises and management consulting firms from all industries and sectors. We continue to provide digital, cloud & AI solutions that enable organizations to enhance, control and streamline their processes while easing the burden of regulatory compliance and quality management programs.
To explore further or discuss how Interfacing can assist your organization, please complete the form below.

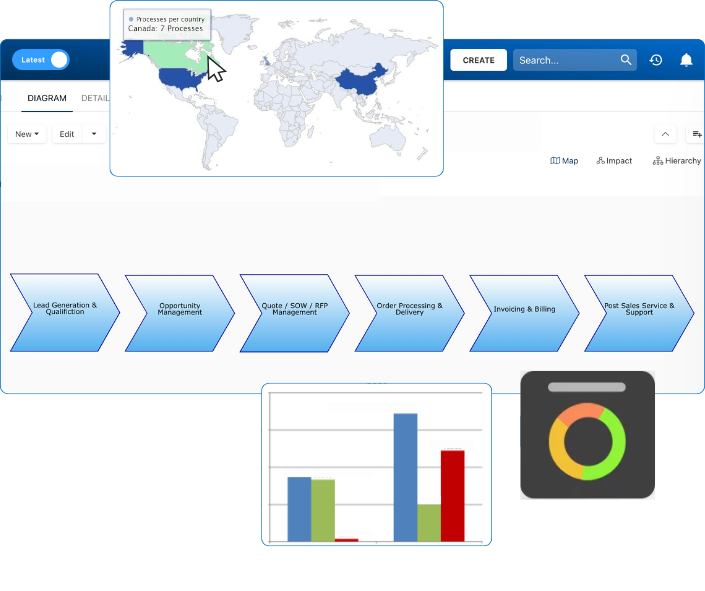
Documentation: Driving Transformation, Governance and Control
• Gain real-time, comprehensive insights into your operations.
• Improve governance, efficiency, and compliance.
• Ensure seamless alignment with regulatory standards.

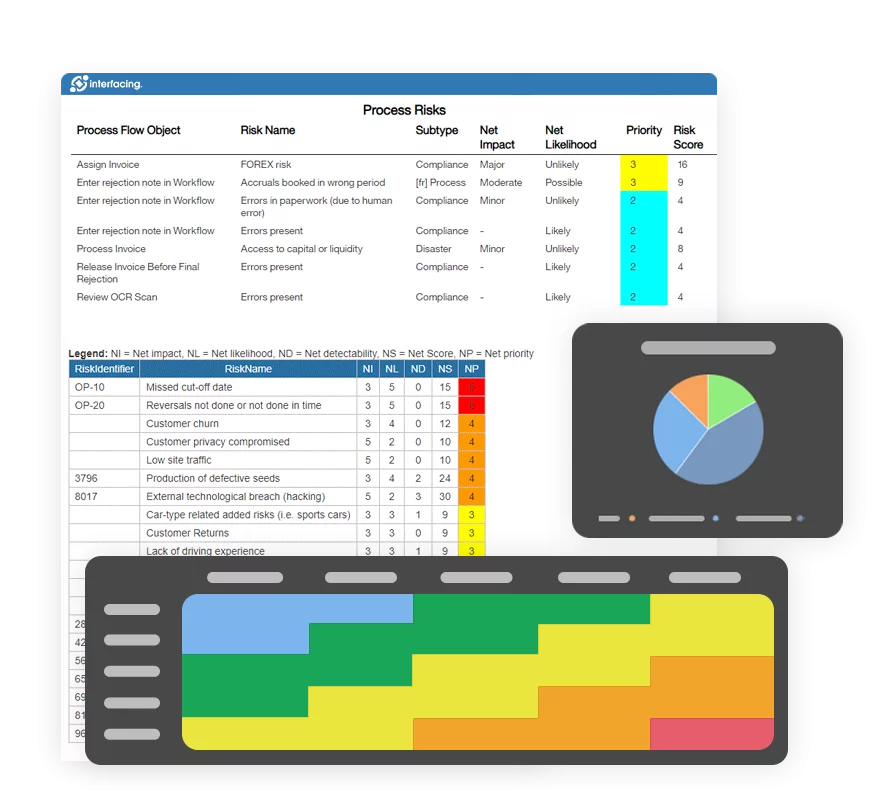
eQMS: Automating Quality & Compliance Workflows & Reporting
• Simplify quality management with automated workflows and monitoring.
• Streamline CAPA, supplier audits, training and related workflows.
• Turn documentation into actionable insights for Quality 4.0

Low-Code Rapid Application Development: Accelerating Digital Transformation
• Build custom, scalable applications swiftly
• Reducing development time and cost
• Adapt faster and stay agile in the face of
evolving customer and business needs.
AI to Transform your Business!
The AI-powered tools are designed to streamline operations, enhance compliance, and drive sustainable growth. Check out how AI can:
• Respond to employee inquiries
• Transform videos into processes
• Assess regulatory impact & process improvements
• Generate forms, processes, risks, regulations, KPIs & more
• Parse regulatory standards into requirements

Request Free Demo
Document, analyze, improve, digitize and monitor your business processes, risks, regulatory requirements and performance indicators within Interfacing’s Digital Twin integrated management system the Enterprise Process Center®!
Trusted by Customers Worldwide!
More than 400+ world-class enterprises and management consulting firms