- Business Process Management (BPM)Document Management System (DMS)Electronic Quality Management System (QMS)Risk, Governance & Compliance (GRC)Low Code Rapid Application Development (LC)Business Continuity Management (BCM)Enterprise Architecture (EA)Business Process Management (BPM)Document Management System (DMS)
- Document Control Overview
- AI Content Creation & Improvement
- Policy & Procedure Management (SOP)
- AI Content Mining Parser
- Collaboration & Governance
- Data Migration & Integration
- Interfacing Offline App
 Electronic Quality Management System (QMS)
Electronic Quality Management System (QMS)- Quality Management System Overview
- Document Control & Records Management
- Audit & Accreditation Management
- Corrective & Preventative Action
- Quality Event (Non-conformity / Complaint/ Compliance)
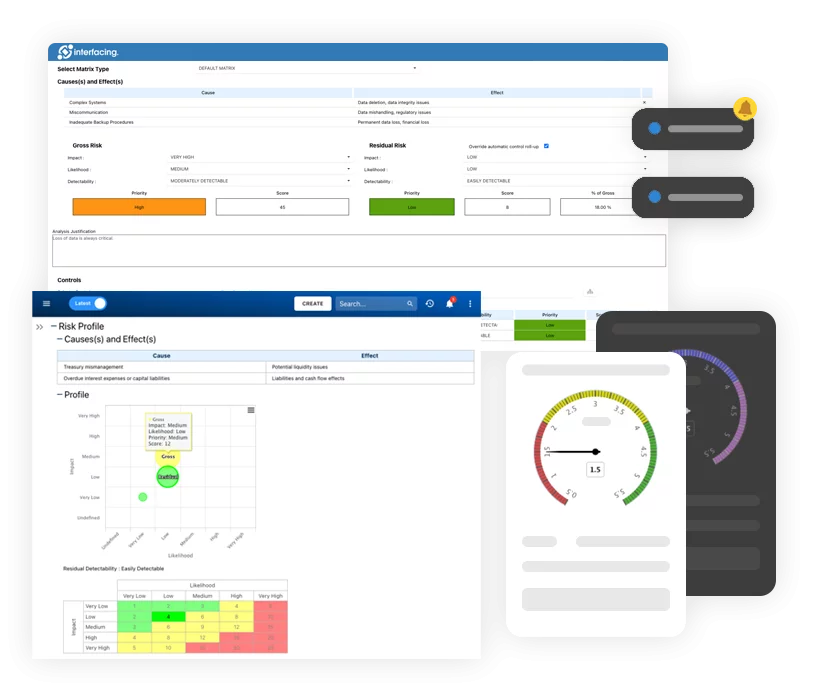
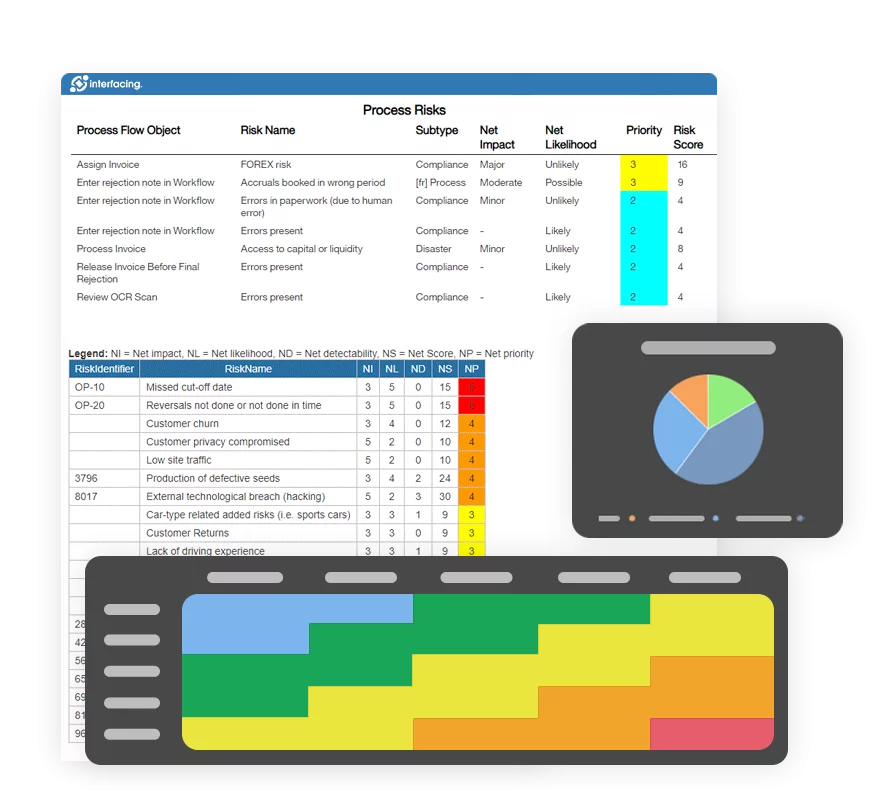
- Risk Management
- Incident Management
- Environmental Health & Safety
- Product & Supplier Management (SCAR)
- Training Management
- Control Management
- Action Items Management
- Management Review
- FMEA
- Pharmacovigilance
- Data Migration & Integration
 Risk, Governance & Compliance (GRC)
Risk, Governance & Compliance (GRC)- Risk, Governance & Compliance Overview
- Risk & Control Management
- Regulatory Compliance
- Collaboration & Governance
- Data Migration & Integration
- Interfacing Offline App
 Low Code Rapid Application Development (LC)
Low Code Rapid Application Development (LC)- Low Code Automation Platform Overview
- Electronic Web Form Design (eFORMS)
- Database Table Entity Designer
- System Integration Designer
- Design & Manage Tasks
- Design & Manage BPMS Apps
- Custom Rules/Guards/Actions
- Electronic Services
- User Homepage
- BAM (Business Activity Monitoring)
- Custom Dashboard Design
- Data Migration & Integration
 Business Continuity Management (BCM)
Business Continuity Management (BCM)- Business Continuity Management Overview
- Business Impact Analysis
- Disaster Recovery Simulation
- Action Item Management
- Mass Notification Management
- Asset Management
- Interfacing Offline App
 Enterprise Architecture (EA)
Enterprise Architecture (EA) - IndustriesRegulatory ComplianceUse CasesLearning CenterFramework & PracticesIndustries
- Healthcare
- Medical Device Technology
- Life Science, Pharmaceutical
- Aerospace & Defense
- Airlines and Aviation
- Media & Telecommunications
- Government and Military
- Technology
- Energy
- Logistics & Port Operations
- Banking & Capital Markets
- Retail & Consumer
- Consulting
- Education
- Engineering & Construction
- Manufacturing
- Financial Services
- Insurance
- Chemicals
Regulatory Compliance- Regulatory Compliance
- ISO
- ISO 9001 (guide)
- ISO 9001:2026 (preparation)
- ISO 17025
- ISO 27000
- ISO 27001
- ISO27002
- ISO 42001
- EU AI Act
- SOC 2 Type 1 & 2
- Sarbanes Oxley
- GxP
- GRC
- Basel
- Digital Signature
- GDPR
- IFRS
- NIST SP 800-53
 Use Cases
Use Cases- Quality Management System (QMS)
- Digital Transformation
- Continuous Improvement
- Governance, Risk & Compliance
- Knowledge Management
- System Deployment (ERP, CRM…)
 Learning CenterFramework & Practices
Learning CenterFramework & Practices - AboutCustomer SuccessPartners

Understanding Eudralex Compliance
Please Select contact form.
Prepare your organization for EU pharmaceutical regulations with digital traceability, AI-powered monitoring, and centralized control.

What is Eudralex?
Eudralex is the official collection of rules and regulations governing medicinal products for human and veterinary use in the European Union.
Its origins trace back to the early 1990s as the EU moved to harmonize pharmaceutical oversight across member states, culminating in a structured legal framework that today spans Good Manufacturing Practice (GMP), Good Clinical Practice (GCP), and Good Distribution Practice (GDP), among others.
Far more than a bureaucratic requirement, Eudralex directly impacts how drugs are developed, tested, manufactured, and monitored across Europe. In an era of global supply chains and increased regulatory scrutiny, compliance with Eudralex isn't just necessary for EU-based firms—it’s mandatory for any company seeking market access within the EU pharmaceutical ecosystem.
Why Eudralex Compliance Matters Today
Eudralex compliance assures regulators, patients, and commercial partners that a product has met stringent safety, efficacy, and quality standards. The consequences of non-compliance can include recalls, product bans, or even criminal liability. Beyond risk mitigation, compliance opens the door to faster market entry, investor confidence, and greater collaboration with EU-based partners.


Real-World Use Case: A U.S. Biotech Expanding to Europe
Background:
A U.S.-based biotech firm developing gene therapies was preparing to enter the EU market via a strategic partnership with a Belgian distributor. While their research met FDA requirements, they lacked any framework aligned with Eudralex GMP or GDP standards. Their documentation was fragmented, and QA processes were heavily manual.
Response:
Rather than building from scratch, the firm deployed Interfacing’s EPC platform to map their current state against Eudralex Volume 4. EPC’s AI engine conducted a gap assessment across documentation, equipment logs, and supplier qualifications. It flagged areas like missing equipment calibration records and inadequate deviation management procedures.
Transformation:
Cross-functional teams collaborated using EPC’s workflow engine to assign ownership, enforce version control, and roll out corrective actions. AI-assisted analytics helped prioritize issues based on inspection risk. Training modules were embedded into the system, with progress logged and reviewed by QPs in real-time.
Outcome:
The firm passed its EU distributor audit with zero critical findings. As a result, it entered the Belgian market on schedule and secured new licensing discussions with German and French partners. EPC continues to monitor deviations and recommend preventive actions based on historical patterns.
Relevant Industries
While Eudralex is centered on pharmaceuticals, its scope influences a much broader ecosystem of regulated industries—each with unique exposure to the framework’s mandates.
Pharmaceutical Manufacturers
These are the primary stakeholders. From initial product development to post-market surveillance, Eudralex outlines every regulatory checkpoint. Firms must manage detailed batch records, implement qualified person (QP) sign-off processes, and maintain manufacturing sites that meet EU GMP requirements. Interfacing’s EPC platform helps pharmaceutical companies digitize audit trails, control SOP changes with automated versioning, and leverage AI to identify risk areas in real time.
Clinical Research Organizations (CROs)
As trials become more complex and decentralized, CROs must comply with GCP standards, particularly Annex 13 and Volume 10 of Eudralex. EPC offers centralized trial master file (TMF) control, AI-driven protocol deviation alerts, and integrated dashboards for sponsor oversight—reducing delays during inspections.
Biotechnology Firms
Biotechs moving from lab to market often underestimate regulatory burdens. Eudralex applies to early-stage innovations as soon as clinical trials begin. Interfacing enables biotech teams to map out regulatory pathways, assign cross-functional responsibilities, and track validation activities with digital workflows.
Medical Device Manufacturers
Though governed by MDR, devices that deliver drugs or interact with pharmaceuticals (e.g., drug-eluting stents) also fall under Eudralex. Using Interfacing’s process governance tools, these firms can model hybrid compliance flows, ensuring both MDR and Eudralex criteria are met simultaneously.
Logistics and Supply Chain Partners
GDP standards in Eudralex Volume 4 apply directly to storage, transportation, and wholesale operations. Interfacing’s EPC can model cold-chain logistics, enforce temperature validation checkpoints, and use AI to flag compliance gaps—ensuring partners meet distributor-level requirements.
Steps to Eudralex Compliance
Perform a Regulatory Gap Analysis
Identify where current practices diverge from Eudralex Volumes (1–10). EPC’s AI automatically flags documentation, process, and oversight gaps.
Define Scope and Responsibilities
Determine which annexes apply and who owns which processes. Use EPC’s organizational charts to align roles with compliance tasks.
Digitize Documentation
Implement a document control system with audit trails, approval workflows, and digital signatures—core requirements under GMP.
Train and Qualify Staff
Launch qualification programs using EPC’s embedded LMS tools. Automate reminders and keep digital logs for inspections.
Audit and Validate
Conduct internal audits mapped to Annex 15 and initiate CAPA workflows. EPC visualizes audit findings and tracks resolution timelines.
Prepare for Inspection
Generate real-time dashboards, SOP libraries, and digital batch records to demonstrate compliance readiness at any moment.

Common Pitfalls to Avoid
Treating Eudralex as a One-Time Task
Compliance isn’t a checklist—it’s a continuous process. EPC’s real-time compliance scoring helps maintain readiness between audits.
Failing to Assign Ownership
Without mapped responsibilities, deviations go unaddressed. EPC enforces role-based accountability through automated workflows.
Using Paper-Based or Fragmented Systems
Regulators expect full traceability. Paper records or legacy spreadsheets increase audit failure risk. EPC unifies your systems and eliminates silos.
Ignoring Changes in Eudralex Updates
Each annex is periodically updated. EPC integrates change management workflows to notify teams and apply updates systematically.
How AI Helps with Eudralex Compliance
Interfacing’s AI capabilities within EPC accelerate compliance by:
Identifying anomalies in manufacturing and audit logs
Recommending targeted CAPAs based on historical trends
Mapping control responsibilities automatically
Monitoring real-time data integrity and flagging compliance drift
With AI doing the heavy lifting, compliance becomes predictive, not reactive.
How Interfacing Can Help
Whether you’re entering the EU market for the first time or improving legacy systems, Interfacing Technologies delivers unmatched visibility and control. Our EPC platform:
Digitizes GMP, GCP, and GDP workflows
Maintains full traceability and audit readiness
Applies AI to reduce manual burden and improve decision-making
Centralizes your entire compliance ecosystem—from training to change management
Let Interfacing simplify your Eudralex journey.

Ensure Process & Quality Governance
Interfacing’s Enterprise Process Center® (EPC) allows you to define, document, and enforce ISO 9001 quality controls organization-wide. Policies, procedures, and SOPs are directly tied to processes and roles—ensuring your QMS is embedded in everyday operations with full transparency. .

Eliminate Manual Errors with AI-Driven QMS
Standardizing documentation and workflows within EPC removes the need for spreadsheets and disconnected systems. Our AI flags outdated procedures, suggests process improvements, and helps maintain version control—reducing the risk of non-compliance and audit issues.

Gain Full Audit Readiness & Traceability
EPC provides real-time traceability across all quality processes—from document approvals to CAPA workflows. With automated audit trails and visual process maps, you’re always ready for certification audits and internal reviews, no last-minute scrambling required

Improve Operational Efficiency Without Sacrificing Compliance
Interfacing’s QMS automates routine tasks such as change control, training sign-offs, and document updates. This not only frees up valuable staff time, but also ensures consistent adherence to ISO 9001 guidelines across all departments and geographies.

Reduce the Cost of Quality Compliance
With centralized process governance and AI-enhanced workflows, EPC reduces the overhead of maintaining your QMS. From smarter audit prep to fewer non-conformities, organizations save time, cut errors, and accelerate their path to ISO 9001 certification.

Build a Culture of Continuous Improvement
ISO 9001 is about more than passing audits—it’s about evolving your organization. Interfacing’s platform helps identify quality gaps, track corrective actions, and promote ongoing learning and accountability—turning compliance into a competitive advantage.
Why Choose Interfacing?
With over two decades of AI, Quality, Process, and Compliance software expertise, Interfacing continues to be a leader in the industry. To-date, it has served over 500+ world-class enterprises and management consulting firms from all industries and sectors. We continue to provide digital, cloud & AI solutions that enable organizations to enhance, control and streamline their processes while easing the burden of regulatory compliance and quality management programs.
To explore further or discuss how Interfacing can assist your organization, please complete the form below.

Documentation: Driving Transformation, Governance and Control
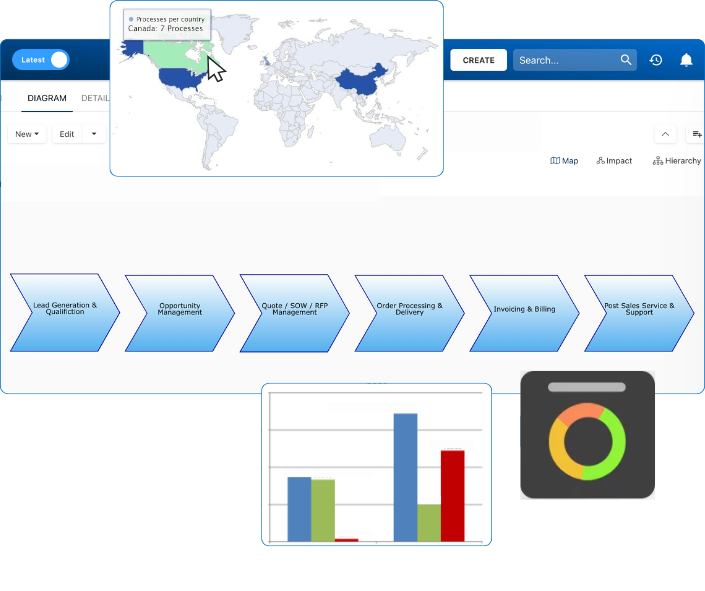
• Gain real-time, comprehensive insights into your operations.
• Improve governance, efficiency, and compliance.
• Ensure seamless alignment with regulatory standards.

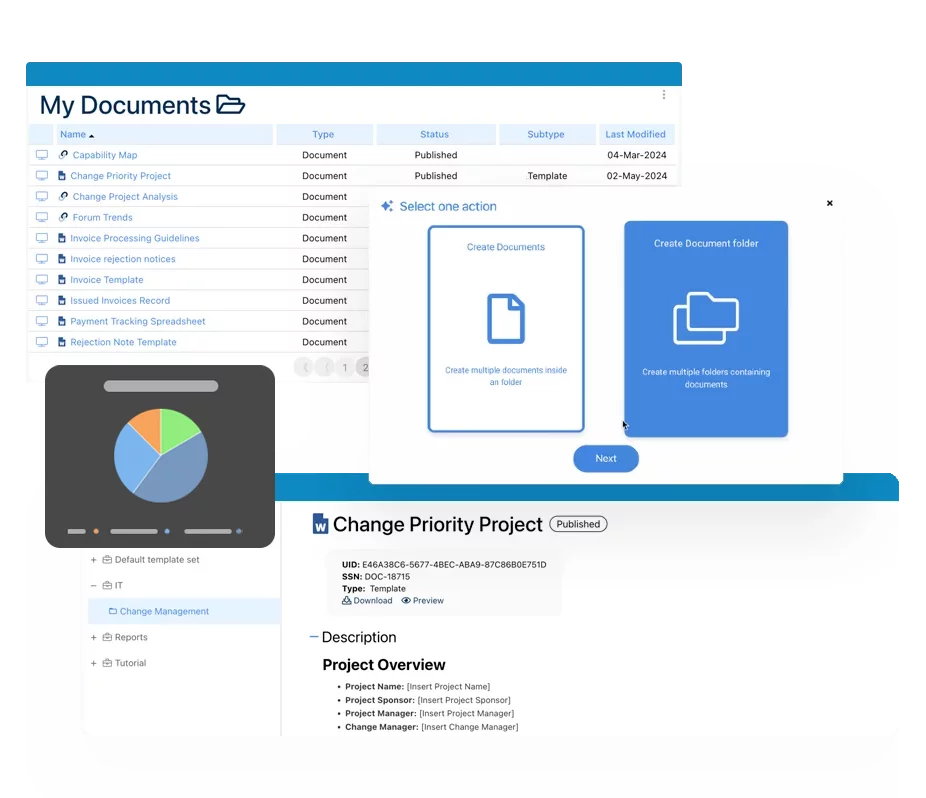
eQMS: Automating Quality & Compliance Workflows & Reporting
• Simplify quality management with automated workflows and monitoring.
• Streamline CAPA, supplier audits, training and related workflows.
• Turn documentation into actionable insights for Quality 4.0

Low-Code Rapid Application Development: Accelerating Digital Transformation
• Build custom, scalable applications swiftly
• Reducing development time and cost
• Adapt faster and stay agile in the face of
evolving customer and business needs.
AI to Transform your Business!
The AI-powered tools are designed to streamline operations, enhance compliance, and drive sustainable growth. Check out how AI can:
• Respond to employee inquiries
• Transform videos into processes
• Assess regulatory impact & process improvements
• Generate forms, processes, risks, regulations, KPIs & more
• Parse regulatory standards into requirements
Request Free Demo
Document, analyze, improve, digitize and monitor your business processes, risks, regulatory requirements and performance indicators within Interfacing’s Digital Twin integrated management system the Enterprise Process Center®!
Trusted by Customers Worldwide!
More than 400+ world-class enterprises and management consulting firms