- Business Process Management (BPM)Document Management System (DMS)Electronic Quality Management System (QMS)Risk, Governance & Compliance (GRC)Low Code Rapid Application Development (LC)Business Continuity Management (BCM)Enterprise Architecture (EA)Business Process Management (BPM)Document Management System (DMS)
- Document Control Overview
- AI Content Creation & Improvement
- Policy & Procedure Management (SOP)
- AI Content Mining Parser
- Collaboration & Governance
- Data Migration & Integration
- Interfacing Offline App
 Electronic Quality Management System (QMS)
Electronic Quality Management System (QMS)- Quality Management System Overview
- Document Control & Records Management
- Audit & Accreditation Management
- Corrective & Preventative Action
- Quality Event (Non-conformity / Complaint/ Compliance)
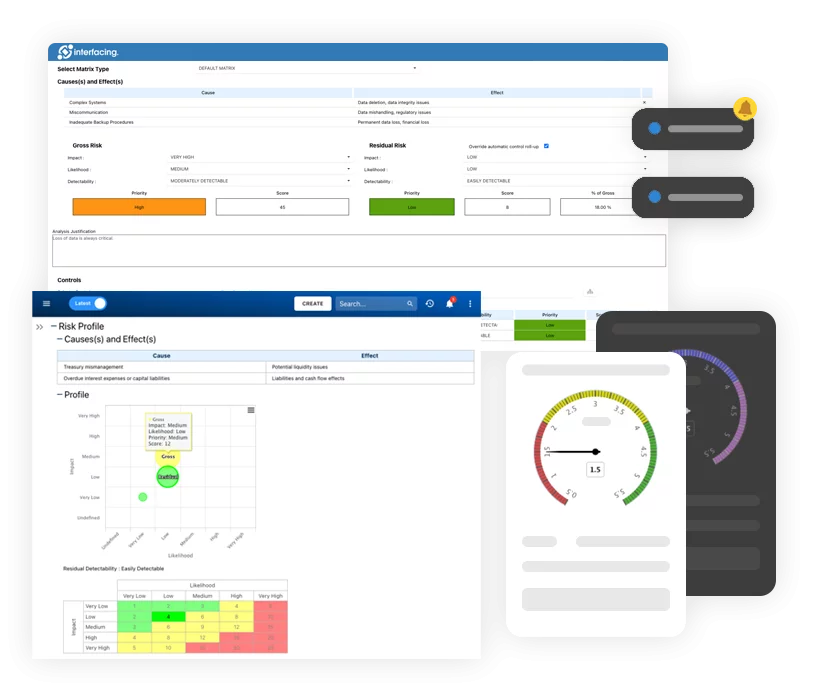
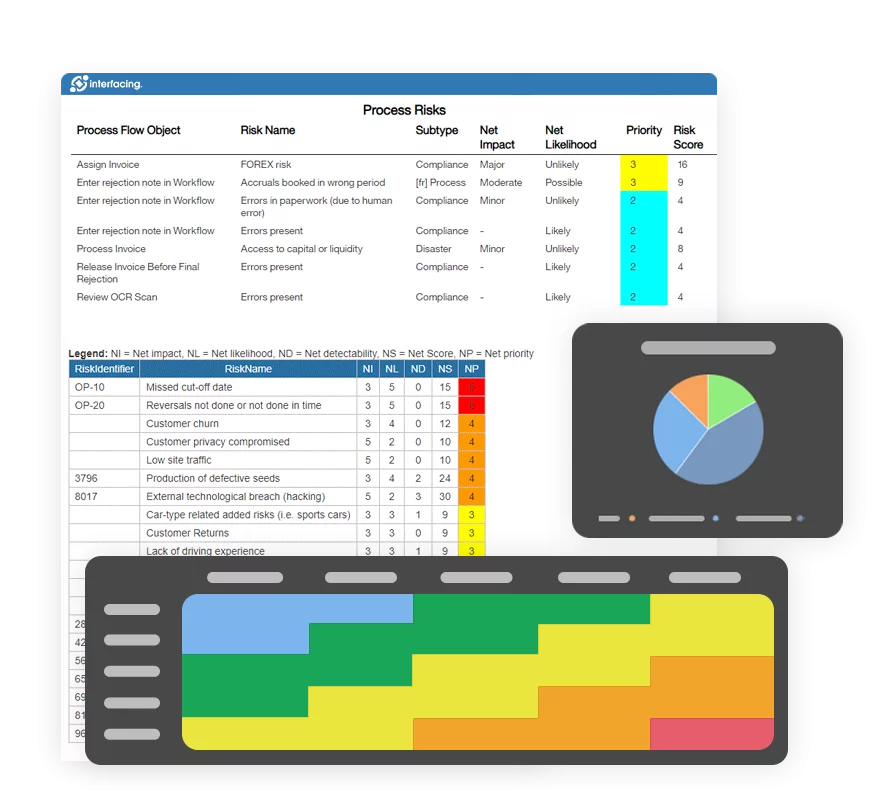
- Risk Management
- Incident Management
- Environmental Health & Safety
- Product & Supplier Management (SCAR)
- Training Management
- Control Management
- Action Items Management
- Management Review
- FMEA
- Pharmacovigilance
- Data Migration & Integration
 Risk, Governance & Compliance (GRC)
Risk, Governance & Compliance (GRC)- Risk, Governance & Compliance Overview
- Risk & Control Management
- Regulatory Compliance
- Collaboration & Governance
- Data Migration & Integration
- Interfacing Offline App
 Low Code Rapid Application Development (LC)
Low Code Rapid Application Development (LC)- Low Code Automation Platform Overview
- Electronic Web Form Design (eFORMS)
- Database Table Entity Designer
- System Integration Designer
- Design & Manage Tasks
- Design & Manage BPMS Apps
- Custom Rules/Guards/Actions
- Electronic Services
- User Homepage
- BAM (Business Activity Monitoring)
- Custom Dashboard Design
- Data Migration & Integration
 Business Continuity Management (BCM)
Business Continuity Management (BCM)- Business Continuity Management Overview
- Business Impact Analysis
- Disaster Recovery Simulation
- Action Item Management
- Mass Notification Management
- Asset Management
- Interfacing Offline App
 Enterprise Architecture (EA)
Enterprise Architecture (EA) - IndustriesRegulatory ComplianceUse CasesLearning CenterFramework & PracticesIndustries
- Healthcare
- Medical Device Technology
- Life Science, Pharmaceutical
- Aerospace & Defense
- Airlines and Aviation
- Media & Telecommunications
- Government and Military
- Technology
- Energy
- Logistics & Port Operations
- Banking & Capital Markets
- Retail & Consumer
- Consulting
- Education
- Engineering & Construction
- Manufacturing
- Financial Services
- Insurance
- Chemicals
Regulatory Compliance- Regulatory Compliance
- ISO
- ISO 9001 (guide)
- ISO 9001:2026 (preparation)
- ISO 17025
- ISO 27000
- ISO 27001
- ISO27002
- ISO 42001
- EU AI Act
- SOC 2 Type 1 & 2
- Sarbanes Oxley
- GxP
- GRC
- Basel
- Digital Signature
- GDPR
- IFRS
- NIST SP 800-53
 Use Cases
Use Cases- Quality Management System (QMS)
- Digital Transformation
- Continuous Improvement
- Governance, Risk & Compliance
- Knowledge Management
- System Deployment (ERP, CRM…)
 Learning CenterFramework & Practices
Learning CenterFramework & Practices - AboutCustomer SuccessPartners

Achieving Global Pharmaceutical Quality with ICH Q10
Please Select contact form.
Understand, implement, and sustain a science-based quality management system aligned with global regulatory expectations.

What is ICH Q10?
The ICH Q10 Pharmaceutical Quality System (PQS) guideline was developed to promote a harmonized approach to pharmaceutical quality management across regulatory regions. Released by the International Council for Harmonisation (ICH), it integrates key elements from GMP regulations, ISO 9001 principles, and lifecycle management into a single global framework.
Rather than focusing solely on compliance, ICH Q10 encourages manufacturers to embed quality into every stage of the product lifecycle—from development to discontinuation.
Today, ICH Q10 plays a pivotal role in regulatory inspections, continuous improvement initiatives, and global submissions. It’s a strategic tool, not just a regulatory checkbox.
Why ICH Q10 is Needed
ICH Q10 fills critical gaps between regulatory compliance and quality innovation. It enables organizations to:
- Reduce variability across global manufacturing operations
- Increase inspection readiness and regulatory trust
- Drive continuous improvement through science and risk-based approaches
Regulators like the FDA and EMA increasingly expect companies to align with ICH Q10 principles—not just adopt local GMPs. For companies seeking market access across jurisdictions, ICH Q10 provides a recognized baseline that supports operational consistency and innovation.


Why ALCOA+ Compliance is Needed
ALCOA+ is critical for ensuring that data used in regulatory submissions, audits, or public health decisions is credible and reproducible. Non-compliance can delay product approvals, damage reputations, or result in enforcement actions. ALCOA+ brings accountability, visibility, and consistency to digital records—whether you're running a clinical trial or managing a production facility.


Real-World Use Case: Global API Supplier Pursues Inspection-Ready Quality
Background:
A global supplier of Active Pharmaceutical Ingredients (APIs), headquartered in Southeast Asia, faced increasing regulatory pressure as they pursued contracts with U.S. and EU clients. Their existing quality system complied with local GMP standards but lacked lifecycle integration, leading to inconsistencies in change control, document traceability, and deviation management.
Response:
The company committed to adopting ICH Q10 as a global standard. Rather than starting from scratch, they partnered with Interfacing to configure the EPC platform as their digital Pharmaceutical Quality System.
Transformation with Interfacing:
The team used EPC to document all stages of the product lifecycle, from development tech transfer to commercial batch release. AI tools flagged incomplete documentation chains and identified role-based gaps in their CAPA process. EPC also automated training matrix updates, tracked QMS ownership by department, and ensured all SOPs met Annex 11 expectations for electronic records.
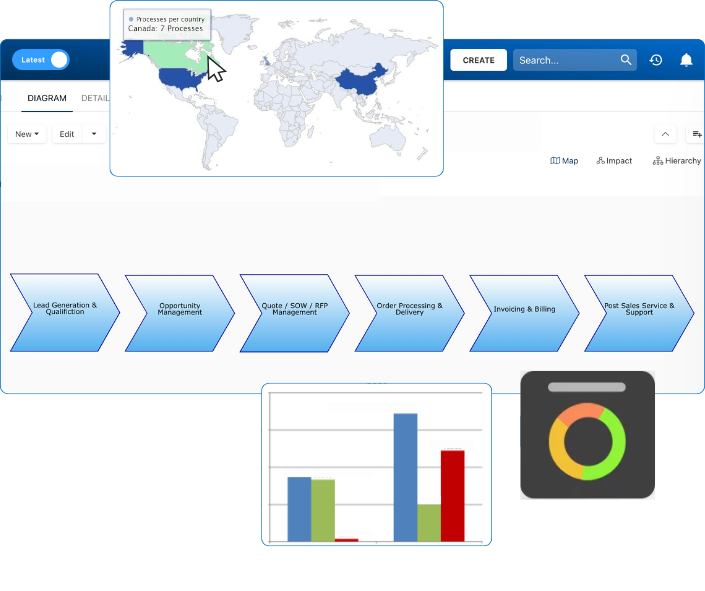
Dashboards offered real-time views of compliance readiness across sites, while internal audits were scheduled and tracked using EPC’s workflow engine.
Outcome:
Within 9 months, the company underwent its first FDA inspection and passed without a Form 483. Clients recognized the maturity of their QMS, opening new revenue opportunities. The EPC platform now supports ongoing improvement cycles and provides alerts for trending risks across product lines.
Relevant Industries
Although ICH Q10 was developed with the pharmaceutical sector in mind, its influence extends to several regulated verticals due to its lifecycle and risk-based principles.
Pharmaceutical Manufacturing
Whether producing solid dose, biologics, or injectables, pharmaceutical firms use ICH Q10 to unify quality oversight across sites and products. From R&D to commercial release, it provides a lifecycle approach to QMS design. Interfacing’s AI-enhanced EPC platform maps quality events across production stages and automates version control, ensuring traceability from lab to label.
Biotechnology
Emerging biotech firms often struggle to scale quality systems during rapid growth. ICH Q10 offers a framework to mature their PQS while maintaining innovation. With EPC, biotech organizations digitize SOPs, assign risk ownership, and receive AI alerts on non-conformities before they escalate.
API Manufacturers and CDMOs
Companies providing active pharmaceutical ingredients or contract development services must demonstrate quality maturity to clients and regulators alike. EPC helps visualize and link critical processes across sites, integrate change management, and align document control to ICH expectations.
Medical Device–Pharma Combinations
For firms producing combination products, ICH Q10 ensures pharmaceutical quality is embedded even when device regulations dominate. EPC integrates compliance documentation across product lines, bridging device and pharma workflows with clarity.
Steps to ICH Q10 Readiness
-
Conduct a Gap Assessment
Evaluate current QMS against ICH Q10 pillars: Process Performance, Product Quality Monitoring, CAPA, Change Management, and Management Review. EPC helps automate this comparison with AI-generated risk maps. -
Define Scope and Responsibilities
Map out lifecycle phases for each product and assign QMS responsibilities using EPC’s org chart and RACI matrix tools. -
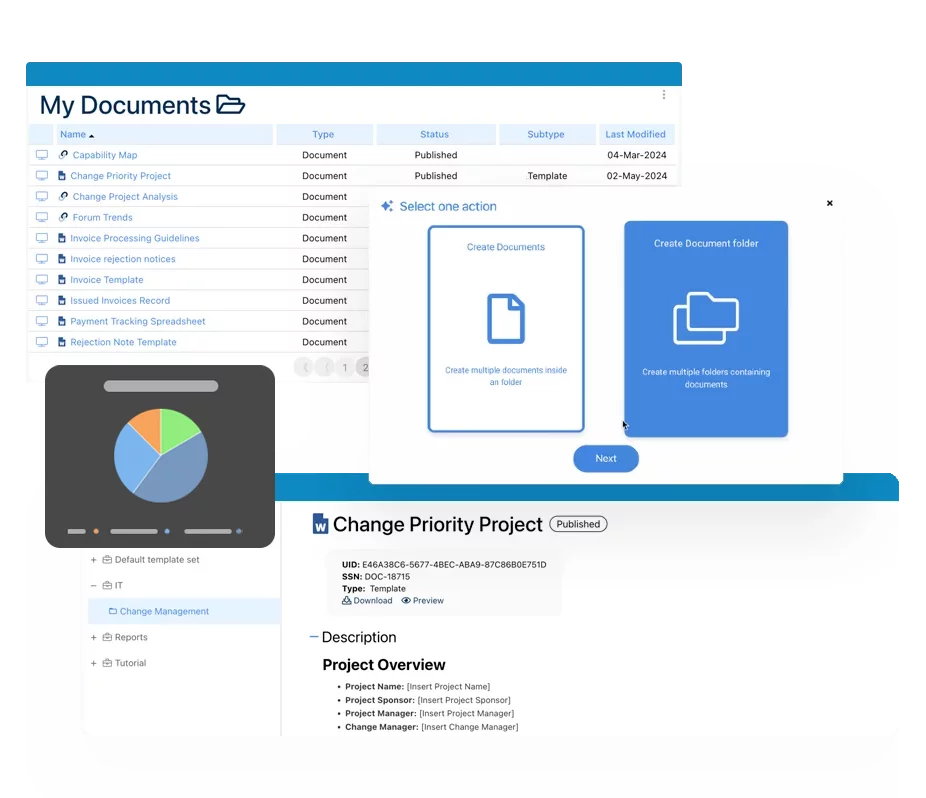
Digitize QMS Documentation
Centralize SOPs, batch records, and training logs. EPC enforces version control, approval routing, and electronic signatures to meet Annex 11/FDA Part 11 standards. -
Launch Change and CAPA Workflows
Build data-driven change control and CAPA workflows aligned with Q10 expectations. EPC ensures escalation rules and timelines are met. -
Engage in Management Reviews and Internal Audits
Use EPC’s dashboards to track QMS KPIs and compliance maturity, and prepare for both internal and regulatory audits with digital audit trails. -
Monitor and Improve Continuously
EPC’s AI scans process data, identifies recurring non-conformities, and recommends updates—transforming QMS from reactive to predictive.

Common Pitfalls to Avoid
-
Treating Q10 as Documentation Only
ICH Q10 requires integration into daily operations—not just updated SOPs. Without AI-enabled monitoring and role enforcement, QMS maturity stalls. -
Siloed QMS Ownership
Quality isn’t the QA department’s job alone. Without mapping ownership across R&D, manufacturing, and supply chain, deviations recur. -
Neglecting Lifecycle Thinking
Many firms apply Q10 only to commercial stages. EPC ensures that tech transfer, process validation, and post-market monitoring are equally governed.
How AI Helps with Eudralex Compliance
Interfacing’s AI capabilities within EPC accelerate compliance by:
Identifying anomalies in manufacturing and audit logs
Recommending targeted CAPAs based on historical trends
Mapping control responsibilities automatically
Monitoring real-time data integrity and flagging compliance drift
With AI doing the heavy lifting, compliance becomes predictive, not reactive.
How Interfacing Helps
Interfacing’s EPC platform serves as the digital backbone for organizations adopting ICH Q10. Our AI-powered tools identify QMS gaps, automate lifecycle workflows, and ensure every compliance element—from training logs to change controls—is traceable, validated, and inspection-ready.
From onboarding to post-implementation audits, Interfacing supports your ICH Q10 journey with visual process modeling, dynamic dashboards, and automated evidence collection. Whether you’re preparing for a global inspection or expanding product lines, EPC provides the confidence and compliance infrastructure you need.

Ensure Process & Quality Governance
Interfacing’s Enterprise Process Center® (EPC) allows you to define, document, and enforce ISO 9001 quality controls organization-wide. Policies, procedures, and SOPs are directly tied to processes and roles—ensuring your QMS is embedded in everyday operations with full transparency. .

Eliminate Manual Errors with AI-Driven QMS
Standardizing documentation and workflows within EPC removes the need for spreadsheets and disconnected systems. Our AI flags outdated procedures, suggests process improvements, and helps maintain version control—reducing the risk of non-compliance and audit issues.

Gain Full Audit Readiness & Traceability
EPC provides real-time traceability across all quality processes—from document approvals to CAPA workflows. With automated audit trails and visual process maps, you’re always ready for certification audits and internal reviews, no last-minute scrambling required

Improve Operational Efficiency Without Sacrificing Compliance
Interfacing’s QMS automates routine tasks such as change control, training sign-offs, and document updates. This not only frees up valuable staff time, but also ensures consistent adherence to ISO 9001 guidelines across all departments and geographies.

Reduce the Cost of Quality Compliance
With centralized process governance and AI-enhanced workflows, EPC reduces the overhead of maintaining your QMS. From smarter audit prep to fewer non-conformities, organizations save time, cut errors, and accelerate their path to ISO 9001 certification.

Build a Culture of Continuous Improvement
ISO 9001 is about more than passing audits—it’s about evolving your organization. Interfacing’s platform helps identify quality gaps, track corrective actions, and promote ongoing learning and accountability—turning compliance into a competitive advantage.
Why Choose Interfacing?
With over two decades of AI, Quality, Process, and Compliance software expertise, Interfacing continues to be a leader in the industry. To-date, it has served over 500+ world-class enterprises and management consulting firms from all industries and sectors. We continue to provide digital, cloud & AI solutions that enable organizations to enhance, control and streamline their processes while easing the burden of regulatory compliance and quality management programs.
To explore further or discuss how Interfacing can assist your organization, please complete the form below.

Documentation: Driving Transformation, Governance and Control
• Gain real-time, comprehensive insights into your operations.
• Improve governance, efficiency, and compliance.
• Ensure seamless alignment with regulatory standards.

eQMS: Automating Quality & Compliance Workflows & Reporting
• Simplify quality management with automated workflows and monitoring.
• Streamline CAPA, supplier audits, training and related workflows.
• Turn documentation into actionable insights for Quality 4.0

Low-Code Rapid Application Development: Accelerating Digital Transformation
• Build custom, scalable applications swiftly
• Reducing development time and cost
• Adapt faster and stay agile in the face of
evolving customer and business needs.
AI to Transform your Business!
The AI-powered tools are designed to streamline operations, enhance compliance, and drive sustainable growth. Check out how AI can:
• Respond to employee inquiries
• Transform videos into processes
• Assess regulatory impact & process improvements
• Generate forms, processes, risks, regulations, KPIs & more
• Parse regulatory standards into requirements
Request Free Demo
Document, analyze, improve, digitize and monitor your business processes, risks, regulatory requirements and performance indicators within Interfacing’s Digital Twin integrated management system the Enterprise Process Center®!
Trusted by Customers Worldwide!
More than 400+ world-class enterprises and management consulting firms