- Business Process Management (BPM)Document Management System (DMS)Electronic Quality Management System (QMS)Risk, Governance & Compliance (GRC)Low Code Rapid Application Development (LC)Business Continuity Management (BCM)Enterprise Architecture (EA)Business Process Management (BPM)Document Management System (DMS)
- Document Control Overview
- AI Content Creation & Improvement
- Policy & Procedure Management (SOP)
- AI Content Mining Parser
- Collaboration & Governance
- Data Migration & Integration
- Interfacing Offline App
 Electronic Quality Management System (QMS)
Electronic Quality Management System (QMS)- Quality Management System Overview
- Document Control & Records Management
- Audit & Accreditation Management
- Corrective & Preventative Action
- Quality Event (Non-conformity / Complaint/ Compliance)
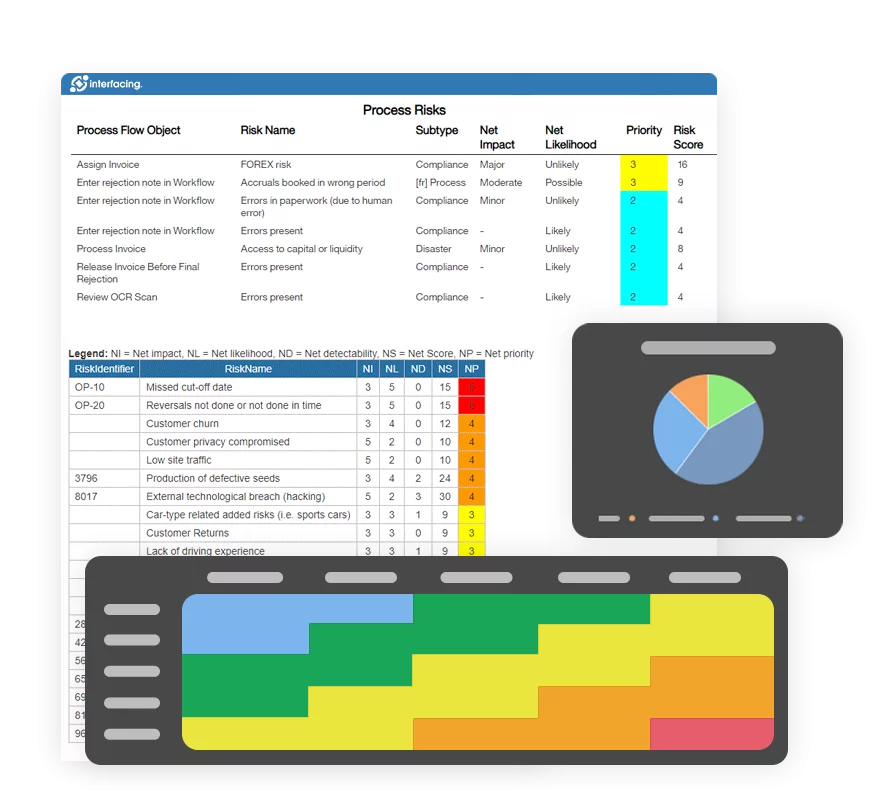
- Risk Management
- Incident Management
- Environmental Health & Safety
- Product & Supplier Management (SCAR)
- Training Management
- Control Management
- Action Items Management
- Management Review
- FMEA
- Pharmacovigilance
- Data Migration & Integration
 Risk, Governance & Compliance (GRC)
Risk, Governance & Compliance (GRC)- Risk, Governance & Compliance Overview
- Risk & Control Management
- Regulatory Compliance
- Collaboration & Governance
- Data Migration & Integration
- Interfacing Offline App
 Low Code Rapid Application Development (LC)
Low Code Rapid Application Development (LC)- Low Code Automation Platform Overview
- Electronic Web Form Design (eFORMS)
- Database Table Entity Designer
- System Integration Designer
- Design & Manage Tasks
- Design & Manage BPMS Apps
- Custom Rules/Guards/Actions
- Electronic Services
- User Homepage
- BAM (Business Activity Monitoring)
- Custom Dashboard Design
- Data Migration & Integration
 Business Continuity Management (BCM)
Business Continuity Management (BCM)- Business Continuity Management Overview
- Business Impact Analysis
- Disaster Recovery Simulation
- Action Item Management
- Mass Notification Management
- Asset Management
- Interfacing Offline App
 Enterprise Architecture (EA)
Enterprise Architecture (EA) - IndustriesRegulatory ComplianceUse CasesLearning CenterFramework & PracticesIndustries
- Healthcare
- Medical Device Technology
- Life Science, Pharmaceutical
- Aerospace & Defense
- Airlines and Aviation
- Media & Telecommunications
- Government and Military
- Technology
- Energy
- Logistics & Port Operations
- Banking & Capital Markets
- Retail & Consumer
- Consulting
- Education
- Engineering & Construction
- Manufacturing
- Financial Services
- Insurance
- Chemicals
Regulatory Compliance- Regulatory Compliance
- ISO
- ISO 9001 (guide)
- ISO 9001:2026 (preparation)
- ISO 17025
- ISO 27000
- ISO 27001
- ISO27002
- ISO 42001
- EU AI Act
- SOC 2 Type 1 & 2
- Sarbanes Oxley
- GxP
- GRC
- Basel
- Digital Signature
- GDPR
- IFRS
- NIST SP 800-53
 Use Cases
Use Cases- Quality Management System (QMS)
- Digital Transformation
- Continuous Improvement
- Governance, Risk & Compliance
- Knowledge Management
- System Deployment (ERP, CRM…)
 Learning CenterFramework & Practices
Learning CenterFramework & Practices - AboutCustomer SuccessPartners

ISO 14001 Environmental Management Compliance
Please Select contact form.
Build a sustainable future and meet global environmental expectations with ISO 14001 certification.
What is ISO 14001?
ISO 14001 is the globally recognized standard for environmental management systems (EMS). First published in 1996 by the International Organization for Standardization, the standard helps organizations reduce their environmental impact, comply with legal and regulatory requirements, and continually improve their environmental performance.
Today, ISO 14001 is more than a badge of eco-friendliness, it’s a strategic asset. It’s used by companies of all sizes and sectors to demonstrate accountability, avoid regulatory fines, and satisfy stakeholder demands around climate impact and sustainability.
Why ISO 14001 is Needed
Regulators, investors, and consumers are demanding stronger environmental accountability. ISO 14001 offers a structured approach to identifying environmental risks, setting improvement objectives, and aligning daily operations with sustainability goals. Whether your motivation is legal compliance, investor confidence, or operational efficiency, ISO 14001 is the trusted global framework.
- Regulatory pressure: Authorities worldwide are tightening environmental laws—ISO 14001 helps organizations stay ahead of evolving compliance requirements.
- Investor expectations: Environmental performance is now a key ESG factor; certification enhances credibility and access to capital.
- Operational gains: A formal EMS reduces waste, lowers costs, and uncovers process inefficiencies that harm both the planet and the bottom line.


Why MHRA GxP Compliance Matters Today
Beyond passing inspections or avoiding fines, GxP compliance reflects an organization’s commitment to public health. It means that products are safe to use, that the data behind those products is trustworthy, and that employees and suppliers are accountable.
For life sciences firms doing business in or with the UK, non-compliance can lead to import bans, suspended operations, or product recalls. But more than that, it can erode trust—with regulators, partners, and patients. GxP is no longer just about documentation. It’s about building a culture of quality where data integrity and process transparency are visible from the inside out.


Who It Applies To—and Why It’s Critical
GxP compliance isn’t confined to traditional pharmaceutical companies. Its reach is broader—and growing.
In biopharma, labs managing preclinical and clinical data must maintain rigorous controls over documentation and electronic signatures, particularly under GCP and GLP. Medical device companies, especially those producing combination products, face MHRA expectations even if they’re already certified under ISO 13485 or UK MDR.
Contract research organizations (CROs) and digital health vendors that manage patient data or clinical workflows are now being held to similar standards, particularly around traceability and audit readiness. Even AI-powered diagnostics and therapeutics fall under scrutiny, especially if their data or models influence clinical outcomes.
What unites these diverse organizations is a shared responsibility: to ensure their processes and records hold up under inspection—and protect end users from harm.


Real-World Use Case: Environmental Risk in Pharmaceuticals
A global pharmaceutical company found itself under increasing scrutiny from regulators and investors. While its quality and safety programs were robust, its environmental practices were fragmented and inconsistent across regions. Incidents of hazardous waste mismanagement and water pollution posed not just regulatory risks, but reputational and financial liabilities.
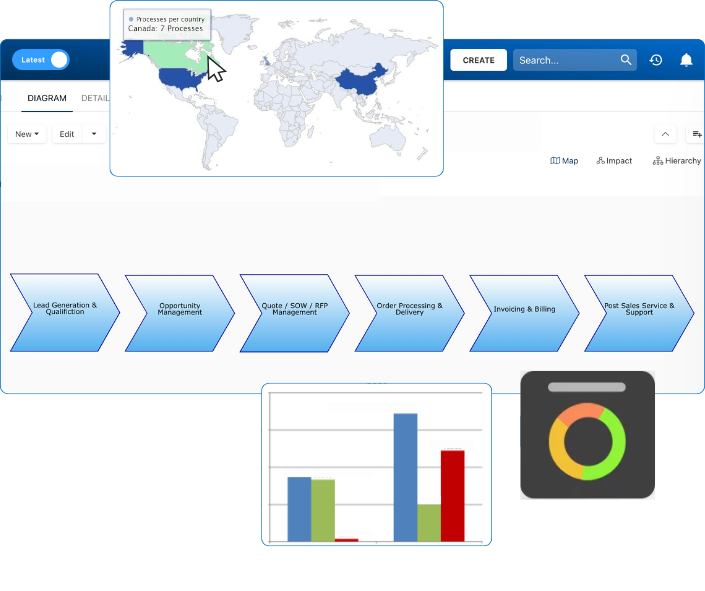
To tackle this, the company adopted ISO 14001 and deployed Interfacing’s Enterprise Process Center (EPC) to digitally map its environmental risks. The initiative began with an enterprise-wide process inventory focused on chemical storage, effluent treatment, waste handling, and water consumption. These were cross-referenced with occupational safety and quality procedures to identify overlaps and gaps.
Interfacing’s AI-driven analytics flagged inconsistencies in local procedures, missing documentation, and underreported compliance incidents. This allowed the leadership team to prioritize high-risk areas and enforce standard controls across all global facilities. Visual dashboards helped each site monitor KPIs in real time, enabling better resource allocation and faster corrective actions.
Within 18 months, the company reported a 32% reduction in hazardous waste incidents, improved internal audit scores, and significantly faster regulatory reporting cycles. The implementation of ISO 14001 not only satisfied compliance requirements—it empowered the organization to embed environmental accountability into every layer of its operations.
Relevant Industries
ISO 14001 applies to nearly every industry, but its greatest impact is felt in sectors with substantial environmental footprints. Whether your organization manages emissions, hazardous waste, or complex supply chains, the standard provides the structure and accountability needed to embed sustainability into daily operations.
Manufacturing organizations use ISO 14001 to establish controls over emissions, chemical handling, and energy consumption. By implementing a formal EMS, manufacturers can reduce waste, avoid costly fines, and strengthen their reputation with environmentally conscious consumers.
Construction and engineering companies rely on ISO 14001 to mitigate site-level pollution, control noise and water runoff, and align with sustainable design principles. The standard is often a prerequisite for green building certifications and major infrastructure bids.
Energy and utility providers face intense scrutiny due to their direct impact on air, water, and land. ISO 14001 helps these organizations monitor environmental performance, manage land use and remediation plans, and demonstrate their commitment to long-term resource stewardship.
Healthcare and life sciences firms benefit from ISO 14001 by implementing safe chemical storage and disposal protocols and environmentally responsible procurement practices. The standard complements existing quality and safety systems, improving transparency and cross-department collaboration.
Financial institutions, while not traditionally viewed as high-footprint, use ISO 14001 to align with ESG reporting frameworks, assess risk across their portfolios, and support sustainability-linked lending strategies. For banks and insurers, the standard adds credibility to environmental governance commitments.
ISO 14001’s flexibility makes it effective for startups and global enterprises alike. Whether you operate a single site or manage a complex international supply chain, the standard provides the blueprint for continuous environmental improvement and regulatory alignment.
Steps to ISO 14001 Certification
Conduct an Environmental Gap Analysis
Evaluate existing processes, documentation, and controls against ISO 14001 requirements. AI tools in EPC assist by scanning for missing procedures and outdated documents.
Define Scope and Environmental Objectives
Identify what parts of your organization and which environmental aspects (e.g., air, water, waste) the EMS will cover. Use process mapping to clarify boundaries.
Document Processes and Assign Responsibilities
Develop or update SOPs for risk controls, compliance monitoring, and operational planning. Assign environmental roles and integrate them into org charts.
Implement Controls and Training
Apply operational and emergency controls, waste handling procedures, and supplier requirements. EPC allows traceability and automatic versioning of documentation.
Monitor and Audit Performance
Use AI to detect deviations and track KPIs in real-time dashboards. Run internal audits and generate improvement logs.
Management Review and Certification Audit
Conduct top-level reviews and engage an accredited body for the certification audit. EPC helps streamline audit prep with centralized logs and evidence libraries.

Common Pitfalls to Avoid
Poor cross-functional involvement
ISO 14001 isn’t just for EHS managers. Without engaging procurement, operations, and HR, your EMS remains siloed.
Neglecting lifecycle thinking
Focusing only on internal impacts misses upstream and downstream risks. ISO 14001 requires considering product and service lifecycles.
Documentation gaps and version confusion
Outdated or inconsistent documents are a top reason for audit failure. EPC enforces digital version control and automatic history tracking.
Failure to track environmental KPIs
Organizations often struggle to define measurable objectives or collect reliable data. AI-driven insights from Interfacing close this gap.
How AI Can Help with ISO 14001
AI makes ISO 14001 more than manageable—it makes it predictive. From real-time emissions tracking to anomaly detection in water or energy usage, Interfacing’s AI-enhanced tools help anticipate risks, streamline data collection, and support decision-making.
With machine learning models embedded into EPC, users can forecast the impact of process changes on compliance targets, detect reporting inconsistencies, and automate audit readiness.
How Interfacing Helps
Interfacing’s Enterprise Process Center (EPC) supports ISO 14001 implementation with:
Visual process mapping for identifying environmental aspects
Centralized document management and automated version control
AI-powered risk and impact analysis
Real-time audit preparation dashboards
Multi-site EMS deployment support
Whether you’re going for first-time certification or re-certifying across regions, Interfacing helps you meet and maintain ISO 14001 requirements efficiently.

Ensure Process & Quality Governance
Interfacing’s Enterprise Process Center® (EPC) allows you to define, document, and enforce ISO 9001 quality controls organization-wide. Policies, procedures, and SOPs are directly tied to processes and roles—ensuring your QMS is embedded in everyday operations with full transparency. .

Eliminate Manual Errors with AI-Driven QMS
Standardizing documentation and workflows within EPC removes the need for spreadsheets and disconnected systems. Our AI flags outdated procedures, suggests process improvements, and helps maintain version control—reducing the risk of non-compliance and audit issues.

Gain Full Audit Readiness & Traceability
EPC provides real-time traceability across all quality processes—from document approvals to CAPA workflows. With automated audit trails and visual process maps, you’re always ready for certification audits and internal reviews, no last-minute scrambling required

Improve Operational Efficiency Without Sacrificing Compliance
Interfacing’s QMS automates routine tasks such as change control, training sign-offs, and document updates. This not only frees up valuable staff time, but also ensures consistent adherence to ISO 9001 guidelines across all departments and geographies.

Reduce the Cost of Quality Compliance
With centralized process governance and AI-enhanced workflows, EPC reduces the overhead of maintaining your QMS. From smarter audit prep to fewer non-conformities, organizations save time, cut errors, and accelerate their path to ISO 9001 certification.

Build a Culture of Continuous Improvement
ISO 9001 is about more than passing audits—it’s about evolving your organization. Interfacing’s platform helps identify quality gaps, track corrective actions, and promote ongoing learning and accountability—turning compliance into a competitive advantage.
Why Choose Interfacing?
With over two decades of AI, Quality, Process, and Compliance software expertise, Interfacing continues to be a leader in the industry. To-date, it has served over 500+ world-class enterprises and management consulting firms from all industries and sectors. We continue to provide digital, cloud & AI solutions that enable organizations to enhance, control and streamline their processes while easing the burden of regulatory compliance and quality management programs.
To explore further or discuss how Interfacing can assist your organization, please complete the form below.

Documentation: Driving Transformation, Governance and Control
• Gain real-time, comprehensive insights into your operations.
• Improve governance, efficiency, and compliance.
• Ensure seamless alignment with regulatory standards.

eQMS: Automating Quality & Compliance Workflows & Reporting
• Simplify quality management with automated workflows and monitoring.
• Streamline CAPA, supplier audits, training and related workflows.
• Turn documentation into actionable insights for Quality 4.0

Low-Code Rapid Application Development: Accelerating Digital Transformation
• Build custom, scalable applications swiftly
• Reducing development time and cost
• Adapt faster and stay agile in the face of
evolving customer and business needs.
AI to Transform your Business!
The AI-powered tools are designed to streamline operations, enhance compliance, and drive sustainable growth. Check out how AI can:
• Respond to employee inquiries
• Transform videos into processes
• Assess regulatory impact & process improvements
• Generate forms, processes, risks, regulations, KPIs & more
• Parse regulatory standards into requirements

Request Free Demo
Document, analyze, improve, digitize and monitor your business processes, risks, regulatory requirements and performance indicators within Interfacing’s Digital Twin integrated management system the Enterprise Process Center®!
Trusted by Customers Worldwide!
More than 400+ world-class enterprises and management consulting firms