Introduction: Why Audit Season Still Sparks Anxiety
Despite years of digitization, many organizations still feel unprepared when audit season approaches. It’s not that they aren’t doing the work—it’s that their tools don’t support audit-readiness as a continuous state. Critical documents are scattered across silos, processes rely too heavily on tribal knowledge, and evidence trails are often reactive rather than real-time.
This scramble is especially familiar in highly regulated sectors like life sciences, aerospace, and finance, where audits aren’t just routine—they’re existential. But it doesn’t have to be this way. With the right connected Quality Management System (QMS), audits shift from panic to proof. And not just proof of compliance, but proof of operational excellence.
Rethinking Compliance: It’s Not Just About Passing the Test
Many organizations still view audits as isolated events, checkpoints they must survive. This mindset leads to last-minute document collection, rushed corrective actions, and hours of “where is that policy?” scavenger hunts. A connected QMS turns this on its head by treating audit readiness as a byproduct of doing business the right way every day.
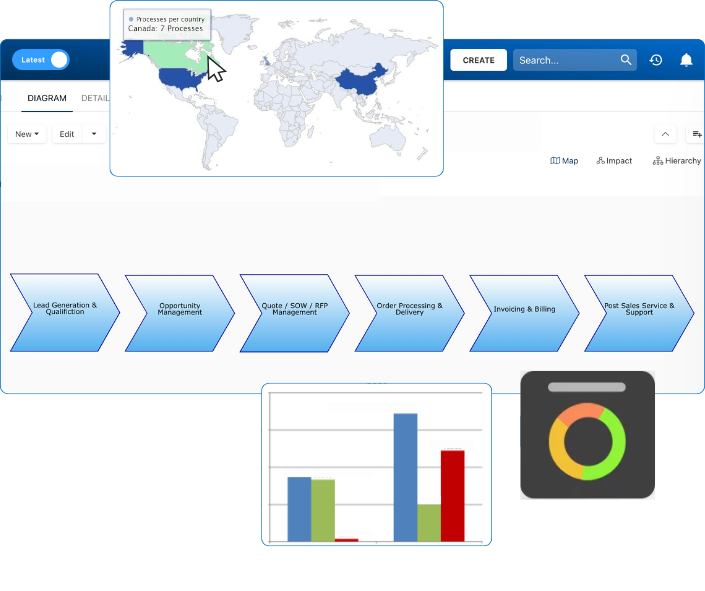
Rather than relying on scattered systems and semi-manual logs, a connected QMS brings everything into one environment: process records, CAPAs, training histories, change requests, and risk assessments. The system captures activity as it happens, builds a digital trail in real time, and links evidence to outcomes. When the auditor comes knocking, the answers are already there.
A Real-World Example: Audit Without the Alarm Bells
Consider a mid-sized pharmaceutical company preparing for a scheduled inspection from the EMA. In previous years, audit prep would mean three weeks of spreadsheet triage and late-night document printing. But after adopting Interfacing’s eQMS platform, their experience changed dramatically.
As the audit approached, the compliance lead was able to pull up digital training logs showing every employee had reviewed the latest SOPs. CAPA reports are linked directly to deviation records. Risk assessments were already mapped to each process. There were no binders, no backtracking, and no last-minute escalations. The audit team walked in and out with confidence and walked away with zero significant findings.
How a Connected QMS Delivers Confidence
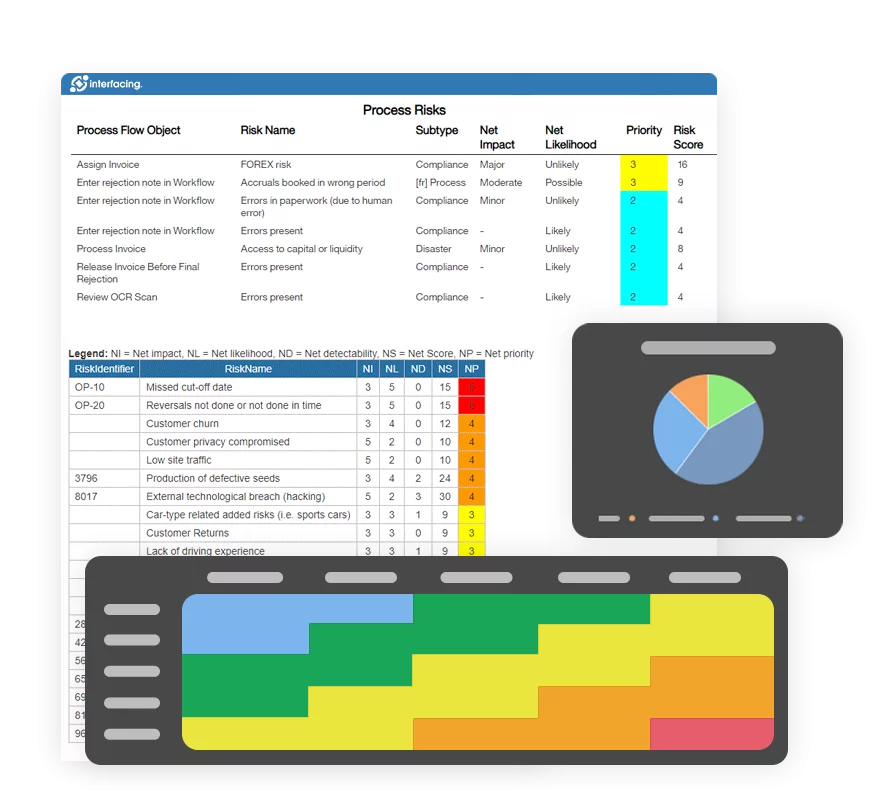
What sets a connected QMS apart is not just its feature set, but the way it reorients your relationship to risk. A well-integrated platform doesn’t wait for non-conformities to bubble up. It surfaces them early. It doesn’t ask users to chase approvals. It routes them intelligently. And it doesn’t let processes stagnate. It makes them visible, measurable, and adaptable.
This is especially vital in industries where oversight is strict and errors are costly. Aerospace companies must maintain AS9100 traceability. Financial firms require robust SOX documentation. Medical device manufacturers need audit-ready logs that comply with FDA 21 CFR Part 11 and EU Annex 11. Interfacing’s platform is built for exactly these realities, validated, secure, and intelligent by design.
Connected Doesn’t Mean Complicated
It’s a common misconception that connecting systems creates complexity. In truth, the proper QMS simplifies it. With Interfacing, you get a centralized environment that supports users without overwhelming them. Version-controlled documents, real-time dashboards, automated notifications, and configurable workflows are all delivered through an intuitive low-code platform.
This means teams aren’t just more compliant, they’re more efficient. Managers can see where training gaps exist. Quality leads can initiate CAPAs directly from event triggers. Executives can monitor audit KPIs without a single spreadsheet. Instead of asking “Are we ready?” the organization knows it is.
The Strategic Payoff of Being Always Audit Ready
There’s a deeper benefit to audit readiness that’s often overlooked: strategic clarity. When your systems are unified and your data is current, you’re no longer playing defense. You have the visibility to prioritize real improvements, the evidence to support change, and the agility to adapt when regulations shift.
Interfacing’s AI-enhanced platform takes this even further. NLP-powered document matching helps identify when SOPs are no longer aligned with changing regulations. Predictive analytics surfaces risks before they become findings. Integrated governance workflows ensure that no compliance task is overlooked.
How Interfacing Can Help
Interfacing’s Digital Integrated Management System (IMS) is engineered for highly regulated environments where audit readiness, process transparency, and data integrity are non-negotiable. Its QMS modules are pre-validated for industries subject to CFR Part 11, GxP, ISO, and more.
Key benefits include:
AI-assisted change impact analysis
Seamless CAPA and audit workflows
Centralized documentation and training records
Embedded version control and e-signatures
Audit trail logging and user access tracking
And because it’s built on a low-code platform, your team can configure workflows and reports without waiting on IT, gaining value faster and adapting more quickly.
Don’t Prepare for Audits. Be Ready for Them.
Audit readiness shouldn’t be a fire drill. It should be a daily outcome of working with the right tools, the right data, and the right mindset. A connected QMS is no longer a luxury—it’s the foundation of operational resilience.
With Interfacing, you don’t just pass audits. You outperform expectations. You build a culture where compliance, quality, and agility work in harmony.
Why Choose Interfacing?
With over two decades of AI, Quality, Process, and Compliance software expertise, Interfacing continues to be a leader in the industry. To-date, it has served over 500+ world-class enterprises and management consulting firms from all industries and sectors. We continue to provide digital, cloud & AI solutions that enable organizations to enhance, control and streamline their processes while easing the burden of regulatory compliance and quality management programs.
To explore further or discuss how Interfacing can assist your organization, please complete the form below.

Documentation: Driving Transformation, Governance and Control
• Gain real-time, comprehensive insights into your operations.
• Improve governance, efficiency, and compliance.
• Ensure seamless alignment with regulatory standards.

eQMS: Automating Quality & Compliance Workflows & Reporting
• Simplify quality management with automated workflows and monitoring.
• Streamline CAPA, supplier audits, training and related workflows.
• Turn documentation into actionable insights for Quality 4.0

Low-Code Rapid Application Development: Accelerating Digital Transformation
• Build custom, scalable applications swiftly
• Reducing development time and cost
• Adapt faster and stay agile in the face of
evolving customer and business needs.
AI to Transform your Business!
The AI-powered tools are designed to streamline operations, enhance compliance, and drive sustainable growth. Check out how AI can:
• Respond to employee inquiries
• Transform videos into processes
• Assess regulatory impact & process improvements
• Generate forms, processes, risks, regulations, KPIs & more
• Parse regulatory standards into requirements

Request Free Demo
Document, analyze, improve, digitize and monitor your business processes, risks, regulatory requirements and performance indicators within Interfacing’s Digital Twin integrated management system the Enterprise Process Center®!
Trusted by Customers Worldwide!
More than 400+ world-class enterprises and management consulting firms