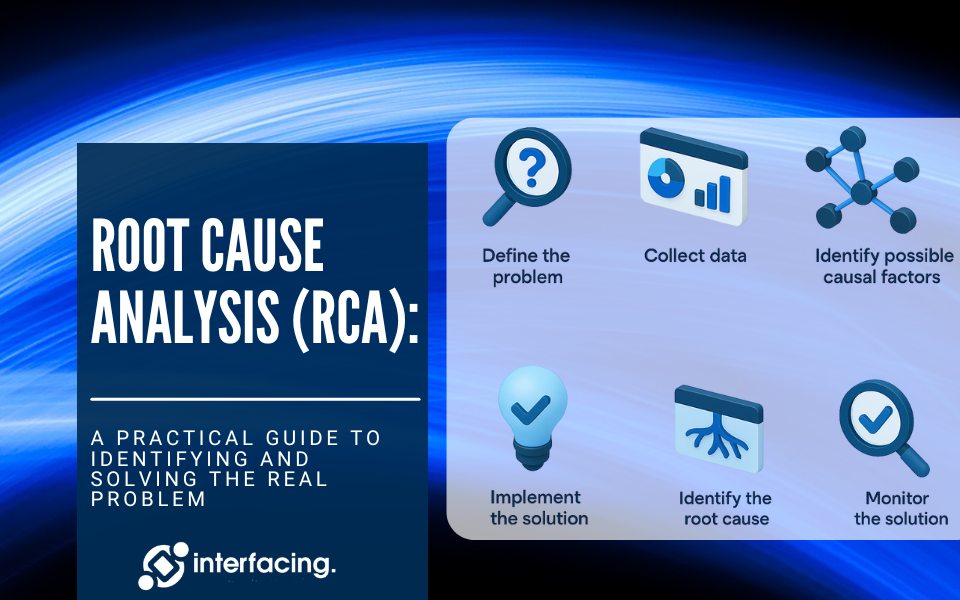
Enterprise Business Process Analysis: What Is It?
Please Select contact form.

According to Gartner®, who coined the term EBPA:
EBPA is the discipline of business and process modeling aimed at transforming and improving business performance with an emphasis on cross-viewpoint, cross-functional analysis to support strategic and operational decisions. EBPA is based on the principles of collaboration, short-cycle delivery, lightweight but robust modeling, and model governance. The two key principles of EBPA are “see the whole” and “understand the value network….” EBPA tools are of interest to business architects, enterprise architects, process architects, process analysts and process owners looking to transform and/or optimize their process-related outcomes.
Introduction
Achieving ISO 9001 certification is a meaningful accomplishment. It signals that your organization has implemented a quality management system (QMS) aligned with internationally accepted standards. But accreditation isn’t the finish line—it’s the baseline.
Many certified companies, especially in aerospace, life sciences, and finance, still struggle with audit failures, unplanned deviations, and inconsistent quality outcomes. Why? Because their QMS, while compliant on paper, is often fragmented, manually updated, and siloed from daily operations.
A digital QMS changes that. It converts static procedures into a dynamic, living system. It enables traceability, enforces workflows, and ensures that compliance is not just documented, but demonstrated in real time.
In this post, we break down the limitations of traditional ISO 9001 implementations and show how digital QMS platforms fill the compliance gaps that lead to costly failures.
Certification Isn’t the Same as Control
Certification confirms that quality processes exist—but it doesn’t guarantee they’re followed consistently. Many companies still rely on a mix of printed SOPs, shared folders, and email approvals. These systems may be enough to pass a scheduled audit, but they often break under pressure or scrutiny.
Real-World Use Case: Aerospace Supplier Audit Breakdown
A Tier 1 aerospace supplier—certified to ISO 9001—relied on a partially digitized QMS. Engineering change requests were submitted via Word forms, approvals collected by email, and SOPs stored in separate SharePoint folders. The system was patched together and depended heavily on employee discipline.
When a major aircraft OEM conducted a supplier audit, they requested documentation on a design change made six months earlier. The supplier couldn’t provide:
A complete audit trail of who approved the change and when,
A linked risk assessment validating the design update, or
Confirmation that the revised SOP was published and acknowledged by production staff.
The audit revealed that the outdated work instruction had remained accessible to the assembly line, resulting in inconsistent outputs across two shifts. This inconsistency triggered a supplier non-conformance report, a Corrective Action Request, and temporary delisting from the client’s approved vendor list.
Had the company implemented a digital QMS:
Change approvals would have been enforced via workflow with mandatory risk assessments.
Document version control would ensure that only the current SOP was accessible,
Training acknowledgments and execution history would be trackable by user ID and timestamp.
This case illustrates a fundamental truth: certification provides structure, but only a digital QMS guarantees control.
Real-Time Visibility Prevents Costly Surprises
ISO 9001 expects organizations to monitor performance and act on data, but that isn’t easy without real-time insights. Most manual QMS tools rely on periodic reviews, meaning compliance teams are always looking backward.
A digital QMS makes it possible to detect and act on issues in real time. Dashboards can show overdue CAPAs, expired training records, uncontrolled documents, and operational bottlenecks. This lets quality and operations leaders respond before small issues escalate.
Real-World Use Case: Biotech Facility Avoids a Costly Deviation
At a mid-sized biotech firm, production line deviations were gradually increasing—first a missed temperature threshold, then a packaging error, then an operator forgetting to log a batch number. None of these seemed urgent on their own, so supervisors logged them manually and moved on.
However, when they implemented a digital QMS with AI-powered monitoring, the system identified a notable trend: all deviations occurred during the night shift and involved the same lot of components from a specific supplier. The root cause? Inadequate lighting and mislabelled parts.
Before the issue could trigger a product recall, the team:
Issued a targeted CAPA,
Adjusted supplier receiving protocols, and
Retrained the night shift team using targeted digital modules.
Without real-time alerts and historical correlation, the pattern would have been missed, and compliance issues could have compounded.
A digital QMS doesn’t just improve visibility. It strengthens preventive action by identifying weak signals early, before they lead to violations or recalls.
Manual CAPA and Change Control Processes Break Under Pressure
Corrective and Preventive Actions (CAPA) and change control are at the heart of ISO 9001’s continuous improvement focus. However, when these processes are disconnected from each other or handled manually, they become sources of delay, confusion, and risk.
Real-World Use Case: Pharmaceutical Manufacturer’s Missed CAPA Escalation
A large pharmaceutical company faced a series of minor but recurring deviations in the cleaning validation process for one of its sterile production lines. Each event was logged and closed locally by the shift lead, but none were escalated because they appeared isolated.
During a regulatory inspection, the auditor identified that five similar deviations had occurred over 18 months, each resolved with a workaround but without any formal investigation. The absence of a CAPA or trend analysis resulted in a Form 483 observation and a follow-up FDA re-inspection.
When the site transitioned to a digital QMS:
All deviations were automatically categorized and trend-monitored,
CAPA workflows triggered escalation when patterns were detected,
Root cause analyses, task assignments, and effectiveness checks were enforced through the system.
The digital QMS not only resolved the immediate issue but also transformed how the company managed operational risks.
Audit Readiness Is a Daily Discipline, Not an Annual Event
Preparing for audits shouldn’t be a fire drill. But for organizations relying on disconnected tools and tribal knowledge, it often is. Last-minute document retrievals, unsigned logs, and missing records are common and damaging.
A digital Quality Management System (QMS) ensures that audit readiness is maintained continuously. Every approval, update, and training completion is time-stamped, versioned, and retrievable instantly.
Real-World Use Case: Medtech Startup Under EU MDR Scrutiny
A medtech startup preparing for EU MDR certification underestimated the rigor of the documentation requirements. Their QMS consisted of well-written procedures, but approvals were stored in email threads, and training records were maintained in Excel.
When the notified body requested a complete audit trail for their software risk assessment, the team had to spend three days manually compiling emails, screenshots, and scanned sign-offs. The process introduced inconsistencies and resulted in an NC (nonconformity) rating on audit follow-up.
Post-audit, the company deployed a digital QMS:
Risk assessments were embedded into the design process with automated traceability.
Digital signatures replaced informal approvals,
Real-time dashboards tracked training completion and document access.
They passed the follow-up inspection with zero findings and are now fully compliant with the EU MDR.
A Digital QMS Builds a Culture of Ownership
Compliance isn’t just about policies—it’s about people. A digital QMS doesn’t replace human accountability; it amplifies it. By making it easier for employees to report issues, follow procedures, and view their responsibilities, it increases engagement and ownership at all levels.
Frontline operators can log non-conformances using guided forms. Engineers can view linked CAPAs and training directly from SOPs. Managers can see completion status and overdue actions in one place. Everyone has access to what they need—when they need it.
When compliance becomes embedded in how work is done, not an extra burden, it becomes sustainable. That’s what separates high-performing organizations from merely compliant ones.
Final Thoughts
ISO 9001 lays the groundwork for quality, but in today’s regulatory and operational environment, it’s not enough. A digital QMS extends the intent of the standard into every workflow, decision, and audit trail. It connects quality to operations. It provides transparency. It enforces follow-through. And it helps you turn compliance into a competitive advantage, not just a certification.
That’s where Interfacing’s EPC Integrated Management System (IMS) stands out. EPC doesn’t just digitize documents—it builds a single source of truth across quality, compliance, and operations. With embedded workflows for CAPA, change control, training, audits, and risk, EPC ensures that ISO 9001 requirements aren’t just met—they’re lived daily across your organization.
For Quality Managers and Operations Directors who need more than a paper shield, EPC offers:
End-to-end visibility with real-time compliance dashboards,
Full lifecycle traceability for documents, deviations, and decisions,
AI-enhanced insights to anticipate risks and drive improvement,
Electronic signatures and validation support to meet FDA/EU requirements,
A centralized, scalable platform ready for ISO 13485, ICH Q10, and beyond.
In regulated industries where the cost of failure is high, EPC empowers teams not only to stay compliant but also to operate with confidence.
Why Choose Interfacing?
With over two decades of AI, Quality, Process, and Compliance software expertise, Interfacing continues to be a leader in the industry. To-date, it has served over 500+ world-class enterprises and management consulting firms from all industries and sectors. We continue to provide digital, cloud & AI solutions that enable organizations to enhance, control and streamline their processes while easing the burden of regulatory compliance and quality management programs.
To explore further or discuss how Interfacing can assist your organization, please complete the form below.

Documentation: Driving Transformation, Governance and Control
• Gain real-time, comprehensive insights into your operations.
• Improve governance, efficiency, and compliance.
• Ensure seamless alignment with regulatory standards.

eQMS: Automating Quality & Compliance Workflows & Reporting
• Simplify quality management with automated workflows and monitoring.
• Streamline CAPA, supplier audits, training and related workflows.
• Turn documentation into actionable insights for Quality 4.0

Low-Code Rapid Application Development: Accelerating Digital Transformation
• Build custom, scalable applications swiftly
• Reducing development time and cost
• Adapt faster and stay agile in the face of
evolving customer and business needs.
AI to Transform your Business!
The AI-powered tools are designed to streamline operations, enhance compliance, and drive sustainable growth. Check out how AI can:
• Respond to employee inquiries
• Transform videos into processes
• Assess regulatory impact & process improvements
• Generate forms, processes, risks, regulations, KPIs & more
• Parse regulatory standards into requirements

Request Free Demo
Document, analyze, improve, digitize and monitor your business processes, risks, regulatory requirements and performance indicators within Interfacing’s Digital Twin integrated management system the Enterprise Process Center®!
Trusted by Customers Worldwide!
More than 400+ world-class enterprises and management consulting firms