- Business Process Management (BPM)Document Management System (DMS)Electronic Quality Management System (QMS)Risk, Governance & Compliance (GRC)Low Code Rapid Application Development (LC)Business Continuity Management (BCM)Enterprise Architecture (EA)Business Process Management (BPM)Document Management System (DMS)
- Document Control Overview
- AI Content Creation & Improvement
- Policy & Procedure Management (SOP)
- AI Content Mining Parser
- Collaboration & Governance
- Data Migration & Integration
- Interfacing Offline App
 Electronic Quality Management System (QMS)
Electronic Quality Management System (QMS)- Quality Management System Overview
- Document Control & Records Management
- Audit & Accreditation Management
- Corrective & Preventative Action
- Quality Event (Non-conformity / Complaint/ Compliance)
- Risk Management
- Incident Management
- Environmental Health & Safety
- Product & Supplier Management (SCAR)
- Training Management
- Control Management
- Action Items Management
- Management Review
- FMEA
- Pharmacovigilance
- Data Migration & Integration
 Risk, Governance & Compliance (GRC)
Risk, Governance & Compliance (GRC)- Risk, Governance & Compliance Overview
- Risk & Control Management
- Regulatory Compliance
- Collaboration & Governance
- Data Migration & Integration
- Interfacing Offline App
 Low Code Rapid Application Development (LC)
Low Code Rapid Application Development (LC)- Low Code Automation Platform Overview
- Electronic Web Form Design (eFORMS)
- Database Table Entity Designer
- System Integration Designer
- Design & Manage Tasks
- Design & Manage BPMS Apps
- Custom Rules/Guards/Actions
- Electronic Services
- User Homepage
- BAM (Business Activity Monitoring)
- Custom Dashboard Design
- Data Migration & Integration
 Business Continuity Management (BCM)
Business Continuity Management (BCM)- Business Continuity Management Overview
- Business Impact Analysis
- Disaster Recovery Simulation
- Action Item Management
- Mass Notification Management
- Asset Management
- Interfacing Offline App
 Enterprise Architecture (EA)
Enterprise Architecture (EA) - IndustriesRegulatory ComplianceUse CasesLearning CenterFramework & PracticesIndustries
- Healthcare
- Medical Device Technology
- Life Science, Pharmaceutical
- Aerospace & Defense
- Airlines and Aviation
- Media & Telecommunications
- Government and Military
- Technology
- Energy
- Logistics & Port Operations
- Banking & Capital Markets
- Retail & Consumer
- Consulting
- Education
- Engineering & Construction
- Manufacturing
- Financial Services
- Insurance
- Chemicals
Regulatory Compliance- Regulatory Compliance
- ISO
- ISO 9001 (guide)
- ISO 9001:2026 (preparation)
- ISO 17025
- ISO 27000
- ISO 27001
- ISO27002
- ISO 42001
- EU AI Act
- SOC 2 Type 1 & 2
- Sarbanes Oxley
- GxP
- GRC
- Basel
- Digital Signature
- GDPR
- IFRS
- NIST SP 800-53
 Use Cases
Use Cases- Quality Management System (QMS)
- Digital Transformation
- Continuous Improvement
- Governance, Risk & Compliance
- Knowledge Management
- System Deployment (ERP, CRM…)
 Learning CenterFramework & Practices
Learning CenterFramework & Practices - AboutCustomer SuccessPartners

21 CFR Part 820 Compliance for Medical Devices
Please Select contact form.
Ensure FDA-compliant design, production, and post-market surveillance with AI-powered quality systems.

What is 21 CFR Part 820?
21 CFR Part 820, also known as the Quality System Regulation (QSR), is a U.S. Food and Drug Administration (FDA) regulation that outlines Current Good Manufacturing Practices (CGMP) for medical device manufacturers. These rules govern the design, control, and traceability of devices to ensure safety and effectiveness throughout their lifecycle.
Originally introduced in 1996 and updated in alignment with ISO 13485:2016, this regulation serves as the backbone of FDA compliance for Class I, II, and III medical devices marketed in the United States.
How AI Is Transforming ISO 13485 Compliance
Manual compliance management is slow, error-prone, and costly. AI helps organizations not just meet compliance—but stay ahead of it.
Here’s how AI accelerates ISO 13485 compliance:
- AI-powered process mining detects bottlenecks in QMS workflows.
- Natural language processing extracts insights from audit reports, nonconformance records, and supplier documents.
- Predictive analytics flag high-risk deviations and prevent regulatory issues.
- Smart forms and dynamic workflows streamline document control, training records, and SOP approvals.
By embedding AI into compliance frameworks, companies improve audit readiness, reduce manual errors, and stay aligned with evolving regulations.


Why is it needed?
The FDA’s 21 CFR Part 820 exists to minimize product recalls, adverse events, and compliance breaches in one of the most highly regulated industries. Without adherence, companies risk warning letters, product seizures, and import bans.
Beyond legal requirements, QSR compliance creates a structured environment for continuous improvement, fostering patient safety and business continuity.


Who Needs to Comply with ISO 13485?
ISO 13485 applies to a broad set of organizations across the medical device supply chain:
- Original Equipment Manufacturers (OEMs)
- Suppliers of components and materials
- Contract sterilization and packaging services
- Developers of software used as a medical device (SaMD)
- Regulatory consultants and authorized representatives

Real-World Use Case
Company:
MedSense Diagnostics – a startup producing AI-powered ECG monitoring patches.
Challenge:
Following market expansion to the U.S., MedSense was hit with a Form 483 citing poor documentation of software validation and noncompliance in design control procedures. The inspection revealed missing links between customer needs and design inputs, along with inconsistent risk analyses for firmware updates.
Solution:
MedSense partnered with Interfacing to deploy an end-to-end digital QMS using the EPC platform.
Process mapping tools were used to trace every step in the design and change process.
AI-enabled audit bots flagged missing validations and incomplete DHFs.
CAPA workflows were embedded directly in SOP documents, ensuring deviations triggered instant root cause workflows.
Training dashboards matched team members’ responsibilities to their latest certifications.
Outcome:
Within six months, the company closed all FDA observations, introduced two new SKUs to market, and secured Series B funding—demonstrating that compliance is not a hurdle but a business enabler.
Relevant Industries
Though 21 CFR Part 820 is a regulation focused on medical devices, its reach extends far beyond traditional manufacturers. Compliance affects a broad ecosystem of businesses involved in design, assembly, software, and logistics in the healthcare value chain.
Medical Device Manufacturers: Any company producing Class I, II, or III devices intended for use in diagnosis, treatment, or prevention must comply. This includes everything from syringes to pacemakers.
Contract Manufacturers (CMOs): Whether producing components or full devices, third-party manufacturers must meet the same quality obligations as OEMs. Many OEMs hold CMOs contractually accountable for QSR adherence.
Software as a Medical Device (SaMD): Companies developing standalone diagnostic software, such as AI-based imaging analysis or mobile health monitoring apps, are increasingly targeted by regulators under Part 820.
In Vitro Diagnostics (IVDs): Blood test kits, PCR machines, and laboratory instruments are expected to comply with design controls and CAPA (Corrective and Preventive Actions) mandates.
Combination Product Developers: Devices that integrate biologics or pharmaceuticals (e.g., prefilled syringes, drug-delivery implants) face dual oversight from device and drug branches of the FDA.
Global Distributors & Importers: Even those who only handle logistics or warehousing may be subject to inspection if they label or repackage devices.
The ecosystem is complex, but aligning with 21 CFR Part 820 builds trust with regulators, payers, and healthcare providers.
Steps to Certification / Inspection Readiness
While the FDA does not issue "certifications," companies must maintain continuous inspection readiness. The journey requires operational maturity, procedural control, and digital oversight. Here’s a comprehensive roadmap:
-
Gap Analysis & Risk Prioritization
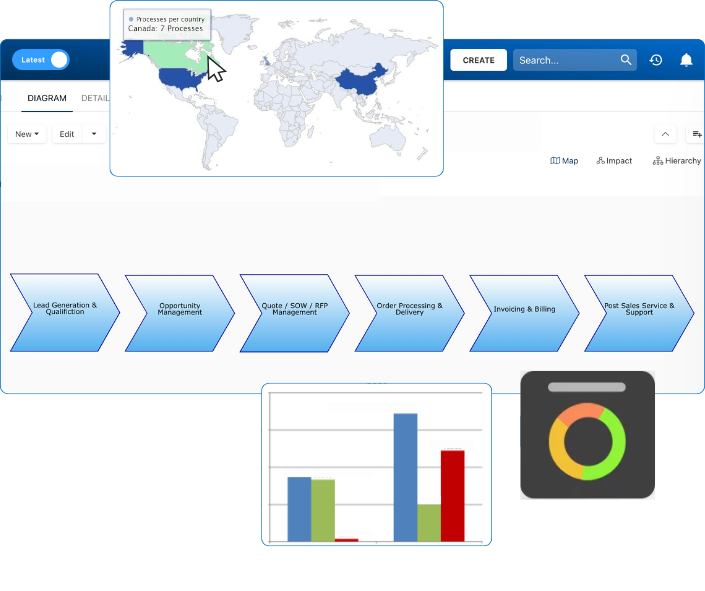
Use AI-powered audit tools to benchmark your current QMS against 21 CFR Part 820 subparts (Design Controls, Production & Process Control, Records, etc.). Interfacing’s EPC platform identifies nonconformities and ranks them by risk, enabling data-driven remediation plans. -
Scope Definition
Clearly define the scope of your QMS: which sites, devices, and suppliers are included. This affects how you document processes, assign roles, and structure audits. With Interfacing, organizations visually map operations across multiple sites and subsidiaries to align compliance with enterprise structure. -
Design and Document Control
Implement robust controls over design planning, inputs, outputs, and verification. AI tools can suggest missing links in design traceability matrices or flag inconsistencies in risk assessments. EPC centralizes documentation and applies digital sign-offs with traceable workflows. -
Training, Roles, and Accountability
Assign compliance roles and ensure employees are properly trained. Many FDA warning letters cite lack of training documentation. Interfacing enables automated learning paths and dashboards that flag skill gaps before audits. -
Internal Audits & Mock Inspections
Schedule internal audits against each QSR section. Use analytics to detect deviations from SOPs and pre-empt audit findings. EPC’s AI audit assistant simulates FDA questions, generates smart CAPAs, and prepares reports for regulatory bodies. -
FDA Inspection / MDSAP Assessment
Prepare for FDA Form 483 outcomes with live dashboards, automated CAPA escalation, and ready-to-export audit trails. Real-time document recall and electronic signatures save critical time during onsite inspections.

Common Pitfalls to Avoid
Achieving compliance isn't just about having policies—it’s about ensuring traceability, accountability, and adaptability under scrutiny. Here are nuanced issues that often result in compliance failures:
Incomplete or Fragmented DHFs
Design History Files must tell a complete, coherent story of how a device evolved from concept to market. Missing documentation or poor version control can invalidate your development timeline. Interfacing’s document governance module ensures audit-ready DHFs with revision history and linked validation protocols.
Overlooking Supplier Controls
Even if your internal QMS is flawless, a supplier’s oversight can lead to major compliance breaches. FDA expects documented supplier qualifications, audits, and controls. Interfacing automates this through digital supplier scoring, performance dashboards, and contract lifecycle integration.
Reactive Instead of Preventive CAPA
Too many firms only act once a deviation occurs. Interfacing’s predictive analytics identify trending failures early—turning reactive systems into proactive QMS environments.
Siloed Training and HR Systems
Disconnected systems lead to gaps between training records and actual job roles. Interfacing provides AI-matched training-to-role matrices with automated expiry alerts and escalation rules.
No Real-Time Risk Monitoring
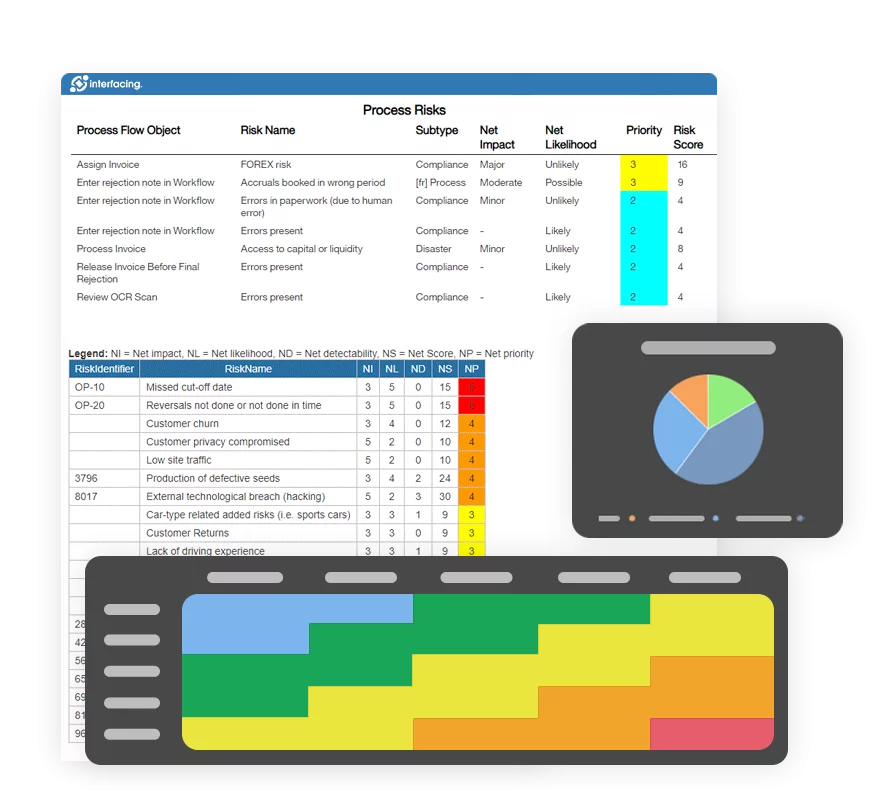
Static risk assessments won’t satisfy auditors. The EPC platform supports dynamic risk registers updated through event triggers (e.g., complaint spikes, audit findings), giving management a real-time view of operational exposure.
How Artificial Intelligence Enhances QMS Compliance
AI transforms traditional compliance into proactive quality governance. Key applications of AI in 21 CFR Part 820 include:
Intelligent Document Control: Automate version management, change approvals, and access logs with natural language scanning and traceability.
Predictive Compliance: AI identifies risk patterns by analyzing deviations, audit findings, or complaint trends before they escalate.
Automated Training Compliance: AI evaluates training completion rates by role, flags overdue certifications, and auto-enrolls employees in re-certification.
Smart CAPA Management: Root cause suggestions based on prior cases and deviation histories accelerate effective corrections.
Regulatory Intelligence: NLP-powered bots scan new FDA guidance and generate compliance impact alerts tailored to your QMS structure.
Interfacing brings AI to every layer of your compliance operation, offering a future-proof advantage.
How Interfacing Can Help
Interfacing Technologies Corporation has helped some of the world’s most regulated companies achieve digital transformation in compliance. For medical device firms aiming to comply with 21 CFR Part 820, we offer:
Visual Process Design
Model and optimize QMS processes using intuitive drag-and-drop tools that map to FDA expectations.Automated Audit & CAPA Tools
Respond to deviations faster with workflows that guide users through root cause, containment, and verification steps.Centralized QMS with AI Support
Unify SOPs, forms, records, and training in a controlled environment—accessible across your global sites.Real-Time Dashboards
Monitor audit readiness, document expiry, employee compliance, and supplier performance from a single interface.
Our platform doesn’t just help you meet compliance—it helps you evolve it.

Ensure Process & Quality Governance
Interfacing’s Enterprise Process Center® (EPC) allows you to define, document, and enforce ISO 9001 quality controls organization-wide. Policies, procedures, and SOPs are directly tied to processes and roles—ensuring your QMS is embedded in everyday operations with full transparency. .

Eliminate Manual Errors with AI-Driven QMS
Standardizing documentation and workflows within EPC removes the need for spreadsheets and disconnected systems. Our AI flags outdated procedures, suggests process improvements, and helps maintain version control—reducing the risk of non-compliance and audit issues.

Gain Full Audit Readiness & Traceability
EPC provides real-time traceability across all quality processes—from document approvals to CAPA workflows. With automated audit trails and visual process maps, you’re always ready for certification audits and internal reviews, no last-minute scrambling required

Improve Operational Efficiency Without Sacrificing Compliance
Interfacing’s QMS automates routine tasks such as change control, training sign-offs, and document updates. This not only frees up valuable staff time, but also ensures consistent adherence to ISO 9001 guidelines across all departments and geographies.

Reduce the Cost of Quality Compliance
With centralized process governance and AI-enhanced workflows, EPC reduces the overhead of maintaining your QMS. From smarter audit prep to fewer non-conformities, organizations save time, cut errors, and accelerate their path to ISO 9001 certification.

Build a Culture of Continuous Improvement
ISO 9001 is about more than passing audits—it’s about evolving your organization. Interfacing’s platform helps identify quality gaps, track corrective actions, and promote ongoing learning and accountability—turning compliance into a competitive advantage.
Why Choose Interfacing?
With over two decades of AI, Quality, Process, and Compliance software expertise, Interfacing continues to be a leader in the industry. To-date, it has served over 500+ world-class enterprises and management consulting firms from all industries and sectors. We continue to provide digital, cloud & AI solutions that enable organizations to enhance, control and streamline their processes while easing the burden of regulatory compliance and quality management programs.
To explore further or discuss how Interfacing can assist your organization, please complete the form below.

Documentation: Driving Transformation, Governance and Control
• Gain real-time, comprehensive insights into your operations.
• Improve governance, efficiency, and compliance.
• Ensure seamless alignment with regulatory standards.

eQMS: Automating Quality & Compliance Workflows & Reporting
• Simplify quality management with automated workflows and monitoring.
• Streamline CAPA, supplier audits, training and related workflows.
• Turn documentation into actionable insights for Quality 4.0

Low-Code Rapid Application Development: Accelerating Digital Transformation
• Build custom, scalable applications swiftly
• Reducing development time and cost
• Adapt faster and stay agile in the face of
evolving customer and business needs.
AI to Transform your Business!
The AI-powered tools are designed to streamline operations, enhance compliance, and drive sustainable growth. Check out how AI can:
• Respond to employee inquiries
• Transform videos into processes
• Assess regulatory impact & process improvements
• Generate forms, processes, risks, regulations, KPIs & more
• Parse regulatory standards into requirements

Request Free Demo
Document, analyze, improve, digitize and monitor your business processes, risks, regulatory requirements and performance indicators within Interfacing’s Digital Twin integrated management system the Enterprise Process Center®!
Trusted by Customers Worldwide!
More than 400+ world-class enterprises and management consulting firms