- Business Process Management (BPM)Document Management System (DMS)Electronic Quality Management System (QMS)Risk, Governance & Compliance (GRC)Low Code Rapid Application Development (LC)Business Continuity Management (BCM)Enterprise Architecture (EA)Business Process Management (BPM)Document Management System (DMS)
- Document Control Overview
- AI Content Creation & Improvement
- Policy & Procedure Management (SOP)
- AI Content Mining Parser
- Collaboration & Governance
- Data Migration & Integration
- Interfacing Offline App
 Electronic Quality Management System (QMS)
Electronic Quality Management System (QMS)- Quality Management System Overview
- Document Control & Records Management
- Audit & Accreditation Management
- Corrective & Preventative Action
- Quality Event (Non-conformity / Complaint/ Compliance)
- Risk Management
- Incident Management
- Environmental Health & Safety
- Product & Supplier Management (SCAR)
- Training Management
- Control Management
- Action Items Management
- Management Review
- FMEA
- Pharmacovigilance
- Data Migration & Integration
 Risk, Governance & Compliance (GRC)
Risk, Governance & Compliance (GRC)- Risk, Governance & Compliance Overview
- Risk & Control Management
- Regulatory Compliance
- Collaboration & Governance
- Data Migration & Integration
- Interfacing Offline App
 Low Code Rapid Application Development (LC)
Low Code Rapid Application Development (LC)- Low Code Automation Platform Overview
- Electronic Web Form Design (eFORMS)
- Database Table Entity Designer
- System Integration Designer
- Design & Manage Tasks
- Design & Manage BPMS Apps
- Custom Rules/Guards/Actions
- Electronic Services
- User Homepage
- BAM (Business Activity Monitoring)
- Custom Dashboard Design
- Data Migration & Integration
 Business Continuity Management (BCM)
Business Continuity Management (BCM)- Business Continuity Management Overview
- Business Impact Analysis
- Disaster Recovery Simulation
- Action Item Management
- Mass Notification Management
- Asset Management
- Interfacing Offline App
 Enterprise Architecture (EA)
Enterprise Architecture (EA) - IndustriesRegulatory ComplianceUse CasesLearning CenterFramework & PracticesIndustries
- Healthcare
- Medical Device Technology
- Life Science, Pharmaceutical
- Aerospace & Defense
- Airlines and Aviation
- Media & Telecommunications
- Government and Military
- Technology
- Energy
- Logistics & Port Operations
- Banking & Capital Markets
- Retail & Consumer
- Consulting
- Education
- Engineering & Construction
- Manufacturing
- Financial Services
- Insurance
- Chemicals
Regulatory Compliance- Regulatory Compliance
- ISO
- ISO 9001 (guide)
- ISO 9001:2026 (preparation)
- ISO 17025
- ISO 27000
- ISO 27001
- ISO27002
- ISO 42001
- SOC 2 Type 1 & 2
- Sarbanes Oxley
- GxP
- GRC
- Basel
- Digital Signature
- GDPR
- IFRS
- NIST SP 800-53
 Use Cases
Use Cases- Quality Management System (QMS)
- Digital Transformation
- Continuous Improvement
- Governance, Risk & Compliance
- Knowledge Management
- System Deployment (ERP, CRM…)
 Learning CenterFramework & Practices
Learning CenterFramework & Practices - AboutCustomer SuccessPartners

MHRA GxP Compliance in the Life Sciences Landscape
Please Select contact form.
Master the expectations of MHRA’s Good Practice (GxP) standards with intelligent compliance tools, digital traceability, and process transparency—powered by AI.

What is MHRA GxP?
When companies talk about GxP compliance, they’re referring to the suite of “Good Practice” standards that ensure product safety, efficacy, and quality in regulated industries. In the United Kingdom, these guidelines are overseen by the Medicines and Healthcare products Regulatory Agency (MHRA).
GMP (Good Manufacturing Practice), GCP (Good Clinical Practice), GDP (Good Distribution Practice), and GLP (Good Laboratory Practice) are the pillars of GxP—each targeting a different stage of the product lifecycle. These aren’t just suggestions. They’re enforceable standards that carry serious implications if ignored.
Since Brexit, MHRA has taken a more independent role in shaping these practices, which means that organizations previously aligned with EU directives must now track evolving UK-specific requirements. This shift underscores the importance of digital readiness and continuous process improvement in achieving—and maintaining—compliance.
How AI Is Transforming ISO 13485 Compliance
Manual compliance management is slow, error-prone, and costly. AI helps organizations not just meet compliance—but stay ahead of it.
Here’s how AI accelerates ISO 13485 compliance:
- AI-powered process mining detects bottlenecks in QMS workflows.
- Natural language processing extracts insights from audit reports, nonconformance records, and supplier documents.
- Predictive analytics flag high-risk deviations and prevent regulatory issues.
- Smart forms and dynamic workflows streamline document control, training records, and SOP approvals.
By embedding AI into compliance frameworks, companies improve audit readiness, reduce manual errors, and stay aligned with evolving regulations.


Why MHRA GxP Compliance Matters Today
Beyond passing inspections or avoiding fines, GxP compliance reflects an organization’s commitment to public health. It means that products are safe to use, that the data behind those products is trustworthy, and that employees and suppliers are accountable.
For life sciences firms doing business in or with the UK, non-compliance can lead to import bans, suspended operations, or product recalls. But more than that, it can erode trust—with regulators, partners, and patients. GxP is no longer just about documentation. It’s about building a culture of quality where data integrity and process transparency are visible from the inside out.


Who It Applies To—and Why It’s Critical
GxP compliance isn’t confined to traditional pharmaceutical companies. Its reach is broader—and growing.
In biopharma, labs managing preclinical and clinical data must maintain rigorous controls over documentation and electronic signatures, particularly under GCP and GLP. Medical device companies, especially those producing combination products, face MHRA expectations even if they’re already certified under ISO 13485 or UK MDR.
Contract research organizations (CROs) and digital health vendors that manage patient data or clinical workflows are now being held to similar standards, particularly around traceability and audit readiness. Even AI-powered diagnostics and therapeutics fall under scrutiny, especially if their data or models influence clinical outcomes.
What unites these diverse organizations is a shared responsibility: to ensure their processes and records hold up under inspection—and protect end users from harm.


Real-World Use Case: Overcoming GxP Gaps During Global Expansion
A mid-sized biotech organization was preparing to launch early-stage clinical trials in a new regulatory jurisdiction governed by MHRA GxP expectations. Internally, they believed they were in good shape. Their quality team had a documented QMS, and training records were available, though spread across spreadsheets, emails, and legacy systems. They had successfully navigated inspections in other regions, so they anticipated few surprises.
The reality proved different.
During a pre-inspection review, regulators identified several critical gaps: missing audit trails for process changes, unclear ownership of quality events, and discrepancies between controlled documents and actual practices on the ground. Worse still, their digital systems hadn’t been validated against GxP standards, and employee training records were inconsistent across departments. What had passed in one market didn’t hold up to MHRA scrutiny.
Facing a six-month delay in trial approval, the company took a hard look at its compliance infrastructure. That’s when they introduced Interfacing’s EPC platform—not just to patch holes, but to rethink their approach to compliance altogether.
The shift was transformative.
Standard operating procedures were digitized and version-controlled with audit trails.
AI flagged missing or outdated training and alerted managers before lapses became findings.
System validation templates were auto-generated and linked to Annex 11 requirements.
Quality events were logged, investigated, and closed out with role-based accountability and digital sign-offs.
Within weeks, the organization had real-time visibility into every compliance indicator across departments. By the time the next inspection came around, their quality team was able to respond to document requests on the spot, with complete traceability, timestamps, and user credentials.
The inspection outcome? Fully compliant, no major observations, and authorization to move forward.
This experience illustrates a broader truth: GxP compliance isn’t static. It evolves with regulatory expectations, operational scale, and the maturity of your systems. Organizations that treat compliance as a living, dynamic process and empower their teams with AI and digital controls are best positioned to thrive, not just pass.
Relevant Industries
MHRA GxP compliance applies to a wide range of industries where health, safety, and product quality are critical. Here’s how it impacts key sectors:
Pharmaceutical and Biotechnology Companies
GxP governs the development, manufacturing, and storage of health products. From batch records to warehousing conditions, compliance ensures operational integrity across the product lifecycle.Clinical Research Organizations (CROs)
These organizations must follow Good Clinical Practice (GCP), particularly when managing sponsor data, electronic documentation, and adverse event reporting. GCP compliance is essential for trial credibility and regulatory approval.Diagnostics and Medical Technology
Labs and device manufacturers rely on Good Laboratory Practice (GLP) to validate test results and guarantee repeatability and data accuracy—core components of product safety and performance.Software and SaaS Vendors
Tools that support regulated workflows, manage quality data, or influence patient outcomes fall under GxP scope. This includes e-signature platforms, digital batch records, and validation systems.Logistics and Cold Chain Providers
Good Distribution Practice (GDP) applies to any organization handling, storing, or transporting regulated goods. These partners must ensure traceability, environmental control, and inspection readiness.
In all of these sectors, GxP compliance isn’t just about passing audits. It’s about building trust in your processes, people, and the products patients depend on.
Steps to MHRA GxP Readiness
Preparing for MHRA GxP compliance means going beyond documentation. It requires a combination of digital visibility, validated systems, and a culture of accountability. Below is a clear progression that organizations typically follow—whether entering a new market or maturing their quality operations.
1. Conduct a GxP Gap Analysis
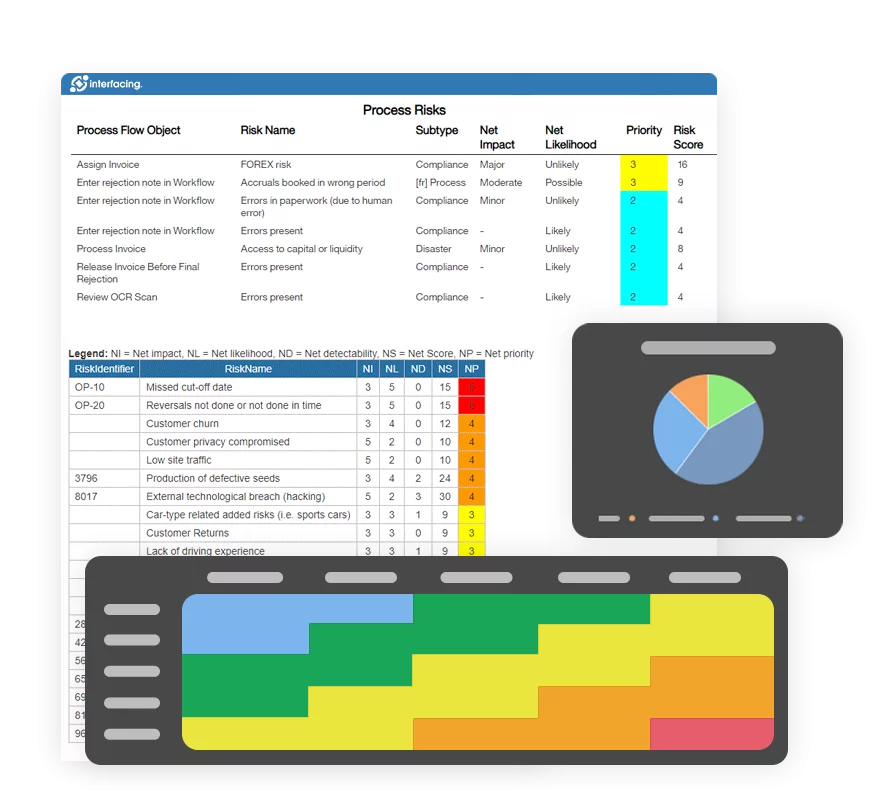
Begin by reviewing current processes, documentation, and systems against applicable GxP standards such as GMP, GLP, GCP, or GDP. AI-driven audit tools—like those in Interfacing’s platform—can accelerate this stage by identifying high-risk areas, missing validations, or procedural inconsistencies that might otherwise go unnoticed.
2. Define Scope and Regulatory Boundaries
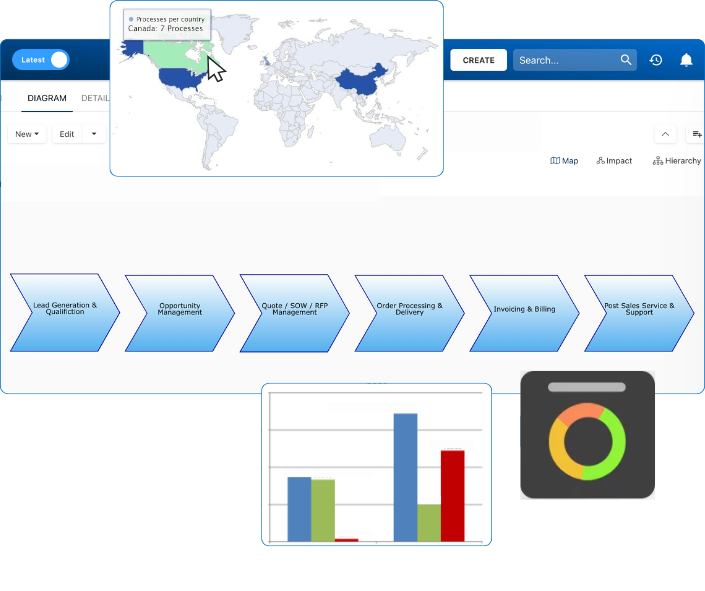
Clearly define what parts of your operations fall under MHRA oversight. This includes identifying which sites, systems, products, and suppliers are subject to GxP controls. An accurate scope helps you allocate resources and avoid misalignment during inspections. Interfacing’s visual process mapping tools help establish this scope across global and multi-site operations.
3. Validate Computerized Systems
Any digital system that stores, modifies, or manages regulated data—spreadsheets included—must be validated according to Annex 11 and data integrity principles. With Interfacing, you can generate and manage validation protocols, maintain audit trails, and control access without relying on manual tracking or disjointed records. Learn more about validation and digital signatures.
4. Centralize SOPs and Controlled Documents
Procedures must be current, version-controlled, and accessible by the right people at the right time. AI can assist by flagging outdated SOPs, highlighting procedural gaps, and ensuring that every process step has a linked policy and responsible owner. With Interfacing, documents are fully integrated with the processes they govern—no silos, no duplication.
5. Train and Track Competency
Regulators expect more than sign-off sheets. They want to see that training is ongoing, relevant, and monitored. Interfacing enables you to auto-assign training based on roles, track completion in real-time, and receive alerts before certifications lapse. AI can even recommend retraining based on audit trends or procedural changes.
6. Establish Ongoing Audit Readiness
MHRA inspections don’t always come with notice. Readiness means having real-time traceability, linked quality events, and closed-loop CAPA systems always available. Interfacing’s dashboards allow quality teams to simulate audit scenarios, generate inspection-ready reports, and drill into root causes—all within one centralized platform.

Common Pitfalls to Avoid
Companies don’t usually fall short due to a lack of effort. It’s often a matter of misalignment. Systems that weren’t designed for regulated environments. Processes that live in someone’s head but never made it to paper. Or paper that was filed but never approved.
One recurring issue is assuming that Annex 11 only applies to big, enterprise-level software. In reality, any digital system—spreadsheets included—must meet validation expectations if it stores, processes, or generates regulated data. AI can assist by generating validation templates, flagging uncontrolled revisions, and verifying access restrictions.
Another pitfall is failing to act on internal audit findings. If your audits don’t lead to documented corrective actions—or if those actions aren’t tracked as they won’t count for much during an MHRA visit.
Ultimately, the most damaging misstep is treating GxP as a one-time event. True compliance is ongoing. It requires systems that update, alert, and adapt. Interfacing helps organizations establish that rhythm with built-in audit trails, AI-generated insights, and continuous monitoring features.
How AI Supports GxP Compliance
GxP compliance isn’t about replacing people with AI—it’s about supporting those people with tools that see what they can’t. Interfacing’s platform uses artificial intelligence to:
Detect risks in real-time
Highlight procedural drift across departments
Correlate audit data with training gaps
Auto-suggest missing links in process maps or SOP trees
In a world where data integrity violations can halt operations, AI isn’t a luxury—it’s an inspection-ready necessity.
How Interfacing Helps
Interfacing’s Integrated Management System was built with GxP in mind. We don’t just digitize your quality system—we make it smarter.
Our clients benefit from:
Centralized documentation linked to each process and role
Audit trails that meet MHRA’s traceability and version control standards
AI that doesn’t just report issues, but anticipates them
Because we support hybrid operations, your remote teams, contract partners, and facilities stay aligned on the same platform, under the same rules.

Ensure Process & Quality Governance
Interfacing’s Enterprise Process Center® (EPC) allows you to define, document, and enforce ISO 9001 quality controls organization-wide. Policies, procedures, and SOPs are directly tied to processes and roles—ensuring your QMS is embedded in everyday operations with full transparency. .

Eliminate Manual Errors with AI-Driven QMS
Standardizing documentation and workflows within EPC removes the need for spreadsheets and disconnected systems. Our AI flags outdated procedures, suggests process improvements, and helps maintain version control—reducing the risk of non-compliance and audit issues.

Gain Full Audit Readiness & Traceability
EPC provides real-time traceability across all quality processes—from document approvals to CAPA workflows. With automated audit trails and visual process maps, you’re always ready for certification audits and internal reviews, no last-minute scrambling required

Improve Operational Efficiency Without Sacrificing Compliance
Interfacing’s QMS automates routine tasks such as change control, training sign-offs, and document updates. This not only frees up valuable staff time, but also ensures consistent adherence to ISO 9001 guidelines across all departments and geographies.

Reduce the Cost of Quality Compliance
With centralized process governance and AI-enhanced workflows, EPC reduces the overhead of maintaining your QMS. From smarter audit prep to fewer non-conformities, organizations save time, cut errors, and accelerate their path to ISO 9001 certification.

Build a Culture of Continuous Improvement
ISO 9001 is about more than passing audits—it’s about evolving your organization. Interfacing’s platform helps identify quality gaps, track corrective actions, and promote ongoing learning and accountability—turning compliance into a competitive advantage.
Why Choose Interfacing?
With over two decades of AI, Quality, Process, and Compliance software expertise, Interfacing continues to be a leader in the industry. To-date, it has served over 500+ world-class enterprises and management consulting firms from all industries and sectors. We continue to provide digital, cloud & AI solutions that enable organizations to enhance, control and streamline their processes while easing the burden of regulatory compliance and quality management programs.
To explore further or discuss how Interfacing can assist your organization, please complete the form below.

Documentation: Driving Transformation, Governance and Control
• Gain real-time, comprehensive insights into your operations.
• Improve governance, efficiency, and compliance.
• Ensure seamless alignment with regulatory standards.

eQMS: Automating Quality & Compliance Workflows & Reporting
• Simplify quality management with automated workflows and monitoring.
• Streamline CAPA, supplier audits, training and related workflows.
• Turn documentation into actionable insights for Quality 4.0

Low-Code Rapid Application Development: Accelerating Digital Transformation
• Build custom, scalable applications swiftly
• Reducing development time and cost
• Adapt faster and stay agile in the face of
evolving customer and business needs.
AI to Transform your Business!
The AI-powered tools are designed to streamline operations, enhance compliance, and drive sustainable growth. Check out how AI can:
• Respond to employee inquiries
• Transform videos into processes
• Assess regulatory impact & process improvements
• Generate forms, processes, risks, regulations, KPIs & more
• Parse regulatory standards into requirements

Request Free Demo
Document, analyze, improve, digitize and monitor your business processes, risks, regulatory requirements and performance indicators within Interfacing’s Digital Twin integrated management system the Enterprise Process Center®!
Trusted by Customers Worldwide!
More than 400+ world-class enterprises and management consulting firms