- Business Process Management (BPM)Document Management System (DMS)Electronic Quality Management System (QMS)Risk, Governance & Compliance (GRC)Low Code Rapid Application Development (LC)Business Continuity Management (BCM)Enterprise Architecture (EA)Business Process Management (BPM)Document Management System (DMS)
- Document Control Overview
- AI Content Creation & Improvement
- Policy & Procedure Management (SOP)
- AI Content Mining Parser
- Collaboration & Governance
- Data Migration & Integration
- Interfacing Offline App
 Electronic Quality Management System (QMS)
Electronic Quality Management System (QMS)- Quality Management System Overview
- Document Control & Records Management
- Audit & Accreditation Management
- Corrective & Preventative Action
- Quality Event (Non-conformity / Complaint/ Compliance)
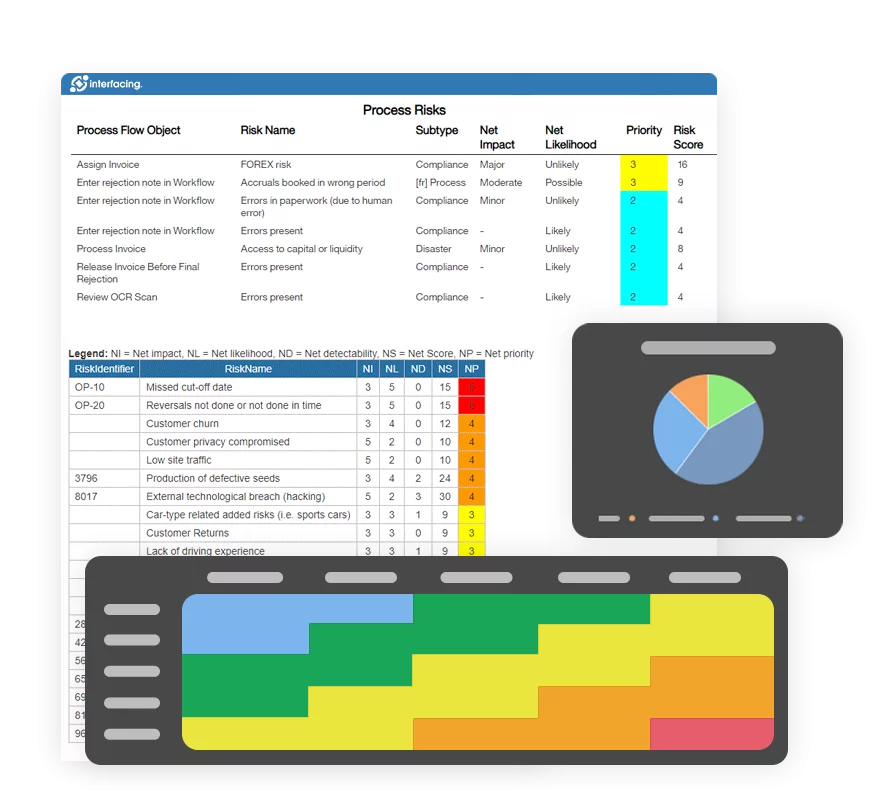
- Risk Management
- Incident Management
- Environmental Health & Safety
- Product & Supplier Management (SCAR)
- Training Management
- Control Management
- Action Items Management
- Management Review
- FMEA
- Pharmacovigilance
- Data Migration & Integration
 Risk, Governance & Compliance (GRC)
Risk, Governance & Compliance (GRC)- Risk, Governance & Compliance Overview
- Risk & Control Management
- Regulatory Compliance
- Collaboration & Governance
- Data Migration & Integration
- Interfacing Offline App
 Low Code Rapid Application Development (LC)
Low Code Rapid Application Development (LC)- Low Code Automation Platform Overview
- Electronic Web Form Design (eFORMS)
- Database Table Entity Designer
- System Integration Designer
- Design & Manage Tasks
- Design & Manage BPMS Apps
- Custom Rules/Guards/Actions
- Electronic Services
- User Homepage
- BAM (Business Activity Monitoring)
- Custom Dashboard Design
- Data Migration & Integration
 Business Continuity Management (BCM)
Business Continuity Management (BCM)- Business Continuity Management Overview
- Business Impact Analysis
- Disaster Recovery Simulation
- Action Item Management
- Mass Notification Management
- Asset Management
- Interfacing Offline App
 Enterprise Architecture (EA)
Enterprise Architecture (EA) - IndustriesRegulatory ComplianceUse CasesLearning CenterFramework & PracticesIndustries
- Healthcare
- Medical Device Technology
- Life Science, Pharmaceutical
- Aerospace & Defense
- Airlines and Aviation
- Media & Telecommunications
- Government and Military
- Technology
- Energy
- Logistics & Port Operations
- Banking & Capital Markets
- Retail & Consumer
- Consulting
- Education
- Engineering & Construction
- Manufacturing
- Financial Services
- Insurance
- Chemicals
Regulatory Compliance- Regulatory Compliance
- ISO
- ISO 9001 (guide)
- ISO 9001:2026 (preparation)
- ISO 17025
- ISO 27000
- ISO 27001
- ISO27002
- ISO 42001
- EU AI Act
- SOC 2 Type 1 & 2
- Sarbanes Oxley
- GxP
- GRC
- Basel
- Digital Signature
- GDPR
- IFRS
- NIST SP 800-53
 Use Cases
Use Cases- Quality Management System (QMS)
- Digital Transformation
- Continuous Improvement
- Governance, Risk & Compliance
- Knowledge Management
- System Deployment (ERP, CRM…)
 Learning CenterFramework & Practices
Learning CenterFramework & Practices - AboutCustomer SuccessPartners

GxP - Good Practices in Pharma
Please Select contact form.
GxP: Compliance guidelines and standards for global pharma

Background of GxP
Life sciences is a heavily regulated environment and one of the most constraining regulation is the set of regulations and guidelines known as
- Good Laboratory Practices (GLP)
- Good Clinical Practices (GCP)
- Good Manufacturing Practices (GMP)
- and others
How Interfacing can help
Regulatory Compliance & Monitoring
Import new regulations and monitor regulatory changes to maintain your library in sync and to trigger a revision workflow.
- Import new regulations through Excel import or via API
- Import regulatory changes through Excel import or via API
- The EPC will automatically generate a new draft version of your regulations, while maintaining your previous regulations
- Regulations and process owners will be notified of changes, and asked to review changes
- Approval cycle is embedded and lifecycle management allows owners to review in-text all changes that were made.
Content Security & Accountability
- Manage your content independently to ensure data safety during the entire lifecycle
- Adapt your approval workflows to embed full accountability through the lifecycle
- Review all changes, all versions of a content fragment in-text, and with visual highlighting of changes.
- Impact Analysis of all changes
- Digital signature verification on all approvals to ensure that secure identity verification (IAMS, Private & Public Keys, Vault, MHRA)
- Multi-factor authentication (MFA) supported, with for example a code being sent to your phone
FDA 21 CFR Part § 11 Compliance
- Accurate and complete copies of records for inspection throughout the records retention period
- Limit access, approvals, and use to authorized individuals and enforce permitted sequencing
- Secure, computer-generated, time-stamped audit trails to record the date of operator entries and actions
- Record changes do not obscure previously recorded information
- Secure and permanent links of all signatures with approver user and date/time information
- Signatures cannot be excised, copied, or otherwise transferred to falsify an electronic record by ordinary means.
- Ensures the authenticity, integrity, and confidentiality of electronic records
- Ensures that each electronic signature is unique
- Employ at least two distinct components + MFA (optional) for each signature for all
- Enforces workflows that doesn’t allow users to bi-pass two distinct component signature authentication within a single session
- Uniqueness of each combined identification code and password
- Employs transaction safeguards to prevent unauthorized use (MFA, Vault)
- Devices that bear or generate identification code or password information are tested
Content Harmonization & Change Management
- Upon publication of a regulation, notifications are issued to all content owners
- If low-impact, then publish changes after impact analysis
- Otherwise, conduct an impact analysis to evaluate impact of changes
- Determine whether impacts are Major or Minor on all connected content (processes, documents, etc.)
- Can trigger individual fragment review processes to ensure that all changes are evaluated individually
- Unless otherwise indicated, changes to downstream content is stricly controlled until the harmonization is completed, validated and approved.
- Content owners are responsible to ensure that all data is harmonized, can visually see highlighted changes and can see content that was flagged as potentially out-of-date.
Cloud-solution for Compliance
As part of our ongoing commitment to compliance and ensuring that our clients meet their regulatory requirements, we are always on the lookout for ways to help our clients attain and maintain full compliance. We are partnering with Amazon Web Services (AWS) for cloud-hosting since their commitment to compliance is second to none. For more information on AWS compliance for GxP, please refer to their compliance program.
Why Choose Interfacing?
With over two decades of AI, Quality, Process, and Compliance software expertise, Interfacing continues to be a leader in the industry. To-date, it has served over 500+ world-class enterprises and management consulting firms from all industries and sectors. We continue to provide digital, cloud & AI solutions that enable organizations to enhance, control and streamline their processes while easing the burden of regulatory compliance and quality management programs.
To explore further or discuss how Interfacing can assist your organization, please complete the form below.

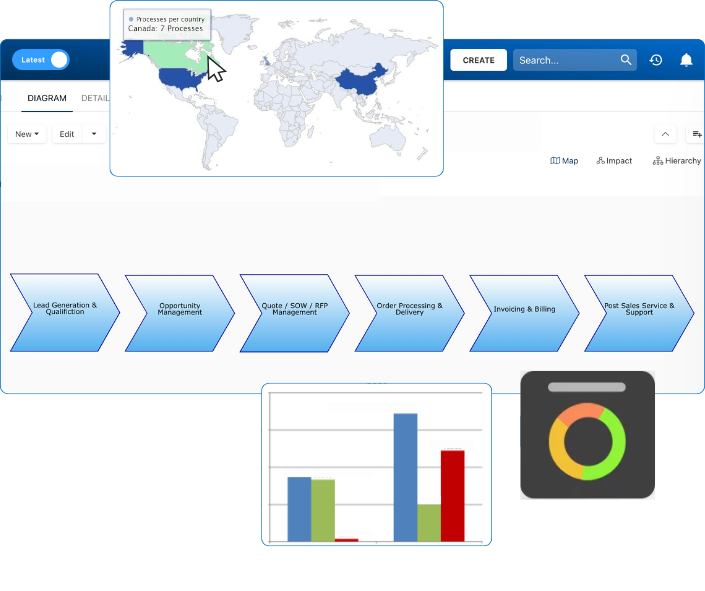
Documentation: Driving Transformation, Governance and Control
• Gain real-time, comprehensive insights into your operations.
• Improve governance, efficiency, and compliance.
• Ensure seamless alignment with regulatory standards.

eQMS: Automating Quality & Compliance Workflows & Reporting
• Simplify quality management with automated workflows and monitoring.
• Streamline CAPA, supplier audits, training and related workflows.
• Turn documentation into actionable insights for Quality 4.0

Low-Code Rapid Application Development: Accelerating Digital Transformation
• Build custom, scalable applications swiftly
• Reducing development time and cost
• Adapt faster and stay agile in the face of
evolving customer and business needs.
AI to Transform your Business!
The AI-powered tools are designed to streamline operations, enhance compliance, and drive sustainable growth. Check out how AI can:
• Respond to employee inquiries
• Transform videos into processes
• Assess regulatory impact & process improvements
• Generate forms, processes, risks, regulations, KPIs & more
• Parse regulatory standards into requirements

Request Free Demo
Document, analyze, improve, digitize and monitor your business processes, risks, regulatory requirements and performance indicators within Interfacing’s Digital Twin integrated management system the Enterprise Process Center®!