- Gestión de Procesos de Negocio (BPM)Sistema de Gestión de Documentos (SGD)Sistema electrónico de gestión de la calidad (SGC)Riesgo, Gobernanza y Cumplimiento (GRC)Desarrollo rápido de aplicaciones de bajo código (LC)Gestión de la Continuidad de Negocio (BCM)Arquitectura Empresarial (EA)Gestión de Procesos de Negocio (BPM)
- Visión general de la gestión de procesos negocio
- Análisis, generación, análisis y mejora de procesos de IA
- Mapeo / Modelado de Procesos
- Análisis y mejora de procesos
- Simulación de procesos
- Minería de procesos
- Colaboración y gobernanza
- Data Migración e integración
- Aplicación offline de Interfacing
 Sistema de Gestión de Documentos (SGD)
Sistema de Gestión de Documentos (SGD)- Visión general del control de documentos
- Creación y mejora de contenidos de IA
- Gestión de políticas y procedimientos (PNT)
- Colaboración y gobernanza
- Data Migración e integración
- Aplicación offline de Interfacing
 Sistema electrónico de gestión de la calidad (SGC)
Sistema electrónico de gestión de la calidad (SGC)- Visión general del sistema de gestión de la calidad
- Control de Documentos y gestión de Registros
- Gestión de Auditoría y Acreditación
- Acción correctiva y preventiva
- Evento de calidad (No conforme/Quejas, Conformidad)
- Gestión de riesgos
- Gestión de incidentes
- Salud y seguridad medioambiental
- Gestión de productos y proveedores (SCAR)
- Gestión de la formación
- Gestión del Control
- Gestión de elementos de acción
- Revisión de la gestión
- FMEA
- Farmacovigilancia
- Data Migración e integración
 Riesgo, Gobernanza y Cumplimiento (GRC)
Riesgo, Gobernanza y Cumplimiento (GRC)- Visión general de Riesgo, Gobernanza y Cumplimiento
- Gestión de riesgos y control
- Cumplimiento de la normativa
- Colaboración y gobernanza
- Data Migración e integración
- Aplicación offline de Interfacing
 Desarrollo rápido de aplicaciones de bajo código (LC)
Desarrollo rápido de aplicaciones de bajo código (LC)- Visión general de la plataforma de automatización Low Code
- Diseño de formularios web electrónicos (eFORMS)
- Visión general de la plataforma de automatización Low Code
- Diseñador de entidades de tabla de base de datos
- Diseñador de Integración de Sistemas
- Seguimiento y delegación de tareas
- Reglas/Guardias/Acciones personalizadas
- Servicios de mensajes de texto/voz
- BAM (Monitorización de la Actividad Empresarial)
- Data Migración e integración
 Gestión de la Continuidad de Negocio (BCM)
Gestión de la Continuidad de Negocio (BCM)- Visión general de BCM
- Análisis del impacto empresarial
- Planificación de la continuidad de la actividad
- Simulación de recuperación en caso de catástrofe
- Gestión de elementos de acción
- Gestión de notificaciones masivas
- Gestión de activos
- Data Migración e integración
 Arquitectura Empresarial (EA)
Arquitectura Empresarial (EA) Consulting Services
Interfacing está aquí para guiarle en cualquier iniciativa de transformación.
- IndustriasCumplimiento de la normativaCasos prácticosCentro de AprendizajeMarco y prácticasIndustrias
- Sanidad
- Tecnología de dispositivos médicos
- Ciencias de la vida, Farmacéutica
- Aeroespacial y Defensa
- Aerolíneas y aviación
- Medios de comunicación y telecomunicaciones
- Gobierno y Ejército
- Tecnología
- Energía
- Logística y Operaciones Portuarias
- Banca y mercados de capitales
- Comercio minorista y consumo
- Consulta
- Educación
- Ingeniería y construcción
- Fabricación
- Servicios financieros
- Seguros
- Productos químicos
Cumplimiento de la normativaCasos prácticosCentro de AprendizajeMarco y prácticas - Sobre nosotrosÉxito del clienteSociosSobre nosotrosÉxito del clienteSocios


Leading QMS Software for Pharmaceutical Companies


QMS Software for Pharmaceutical Companies
Please Select contact form.
AI-Powered Quality, Compliance & GxP Automation for Pharma

Unify processes, documentation, and compliance across the product lifecycle
Pharmaceutical organizations face constant pressure to maintain compliance with GxP, FDA 21 CFR Part 11, EU GMP Annex 11, and ICH Q10 while accelerating innovation and protecting patient safety.
Manual document control and disconnected systems can no longer keep up with the pace of regulatory change, global manufacturing, and digital transformation.
Interfacing’s AI-powered Quality Management System (QMS) helps pharmaceutical manufacturers unify processes, documentation, and compliance across the product lifecycle, from research and development to post-market vigilance.
The Challenge: Complexity in a Fast-Moving Digital World
In today’s hyper-connected economy, technology and media organizations must continuously adapt, integrating new tools, updating policies, and ensuring uninterrupted service delivery.
Manual governance processes and fragmented systems often lead to duplication, shadow IT, and non-compliance with frameworks like ISO 27001, GDPR, and SOC 2.
To maintain control at scale, enterprises need a single source of truth that aligns strategy, processes, and security controls, powered by AI for visibility and continuous improvement.


Why Leading Pharma Companies Choose Interfacing
Proven GxP Compliance: Digitally enforce 21 CFR Part 11, Annex 11, and ICH Q10 requirements.
End-to-End Traceability: Link every record, SOP, and CAPA to its related process and regulation.
AI-Powered Insight: Detect non-conformities, automate CAPA, and forecast recurring issues.
Digital Twin of the Organization (DTO): Visualize the full pharma value chain for smarter quality decisions.
Validated Environment: 21 CFR Part 11 / Annex 11–ready — audit trail, electronic signature, and version control.
Request a personalized demo of the AI-Integrated Management System (IMS) to see how global pharma leaders modernize their quality and compliance frameworks.
Streamline Your Pharmaceutical Quality Operations
With Interfacing’s integrated platform, you can:
Manage deviations, CAPA, and change control through automated workflows.
Digitize training management to ensure employee readiness and procedural adherence.
Simplify supplier qualification and audit readiness across your global network.
Ensure real-time visibility with compliance dashboards and KPIs.
Explore related resources:
→ FDA 21 CFR Part 820 Compliance
→ ISO 13485 Compliance Guide
→ GxP Risk Management & Compliance

How Interfacing Helps Pharmaceutical Organizations
Interfacing supports global pharma companies with:
Unified document, process, and risk management
AI-based process discovery for continuous improvement
Integrated quality event and CAPA lifecycle tracking
Secure, validated e-signature and access control
Multi-site, multi-language collaboration for regulated operations
Learn more about the AI-Integrated Management System or schedule a demo with our compliance experts.

Ensure Process & Quality Governance
Interfacing’s Enterprise Process Center® (EPC) allows you to define, document, and enforce ISO 9001 quality controls organization-wide. Policies, procedures, and SOPs are directly tied to processes and roles—ensuring your QMS is embedded in everyday operations with full transparency. .

Eliminate Manual Errors with AI-Driven QMS
Standardizing documentation and workflows within EPC removes the need for spreadsheets and disconnected systems. Our AI flags outdated procedures, suggests process improvements, and helps maintain version control—reducing the risk of non-compliance and audit issues.

Gain Full Audit Readiness & Traceability
EPC provides real-time traceability across all quality processes—from document approvals to CAPA workflows. With automated audit trails and visual process maps, you’re always ready for certification audits and internal reviews, no last-minute scrambling required

Improve Operational Efficiency Without Sacrificing Compliance
Interfacing’s QMS automates routine tasks such as change control, training sign-offs, and document updates. This not only frees up valuable staff time, but also ensures consistent adherence to ISO 9001 guidelines across all departments and geographies.

Reduce the Cost of Quality Compliance
With centralized process governance and AI-enhanced workflows, EPC reduces the overhead of maintaining your QMS. From smarter audit prep to fewer non-conformities, organizations save time, cut errors, and accelerate their path to ISO 9001 certification.

Build a Culture of Continuous Improvement
ISO 9001 is about more than passing audits—it’s about evolving your organization. Interfacing’s platform helps identify quality gaps, track corrective actions, and promote ongoing learning and accountability—turning compliance into a competitive advantage.
¿Por qué elegir Interfacing?
Con más de dos décadas de experiencia en software de IA, Calidad, Procesos y Cumplimiento, Interfacing sigue siendo líder en el sector. Hasta la fecha, ha prestado servicio a más de 500 empresas de talla mundial y consultoras de gestión de todas las industrias y sectores. Seguimos ofreciendo soluciones digitales, en la nube y de IA que permiten a las organizaciones mejorar, controlar y agilizar sus procesos, al tiempo que alivian la carga de los programas de cumplimiento normativo y gestión de la calidad.
Para obtener más información o hablar sobre cómo Interfacing puede ayudar a su organización, rellene el siguiente formulario.

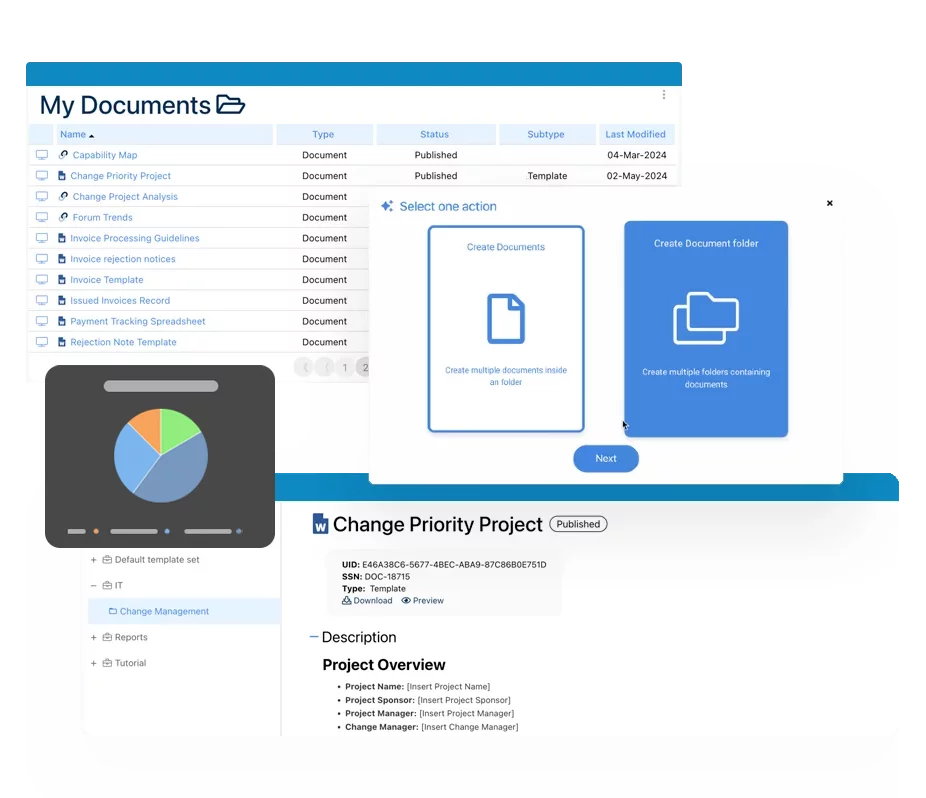
Documentación: Impulsando la Transformación, Gobernanza y Control
• Obtenga información integral en tiempo real sobre sus operaciones.
• Mejore la gobernanza, eficiencia y cumplimiento.
• Garantice la alineación fluida con los estándares regulatorios.

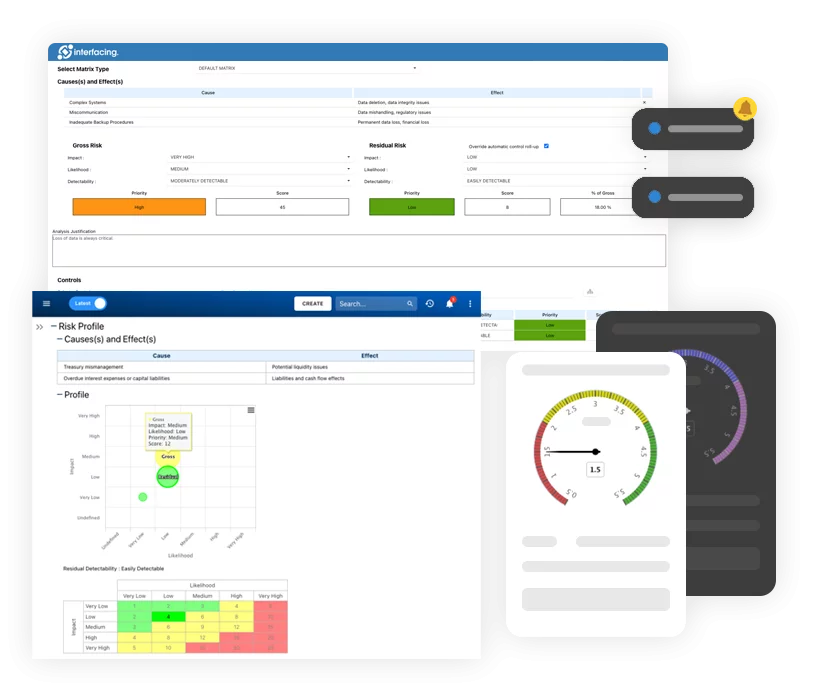
eQMS: Automatización de flujos de trabajo y reportes de calidad y cumplimiento
• Simplifique la gestión de calidad con flujos de trabajo automatizados y monitoreo..
• Optimice CAPA, auditorías de proveedores, capacitaciones y flujos relacionados..
• Transforme la documentación en información procesable para Calidad 4.0.
.

Desarrollo rápido de aplicaciones low-code: Acelerando la transformación digital
• Cree aplicaciones personalizadas y escalables de forma ágil.
• Reduzca el tiempo y costo de desarrollo.
• Adáptese rápidamente y manténgase ágil frente a las necesidades cambiantes de clientes y negocios.

¡IA para transformar su negocio!
Las herramientas impulsadas por IA están diseñadas para optimizar operaciones, mejorar el cumplimiento y fomentar el crecimiento sostenible. Descubra cómo la IA puede:
• Responder a las consultas de los empleados.
• Transformar videos en procesos.
• Formular recomendaciones sobre el impacto de la regulación y la mejora de los procesos
• Generar formularios electrónicos, procesos, riesgos, regulaciones, KPIs y mucho más.
• Desglosar estándares regulatorios en requisitos desagregados.
Request Free Demo
Document, analyze, improve, digitize and monitor your business processes, risks, regulatory requirements and performance indicators within Interfacing’s Digital Twin integrated management system the Enterprise Process Center®!
Con la confianza de Clientes en todo el mundo
Más de 400+ empresas y consultoras de gestión de talla mundial



















































Integración
Con la confianza de Clientes en todo el mundo Integración
Más de 400+ empresas y consultoras de gestión de talla mundial