- Geschäftsprozessmanagement (BPM)Dokumenten-Management-System (DMS)Elektronisches Qualitätsmanagementsystem (QMS)Risk, Governance & Compliance (GRC)Schnelle Anwendungsentwicklung mit wenig Code (LC)Betriebliches Kontinuitätsmanagement (BCM)Unternehmensarchitektur (EA)Geschäftsprozessmanagement (BPM)
- Geschäftsprozessmanagement Übersicht
- AI Prozess-Parsing, -Generierung, -Analyse & -Verbesserung
- Prozess-Mapping / Modellierung
- Prozess-Analyse und Verbesserung
- Prozess-Simulation
- Prozess-Mining
- Zusammenarbeit & Governance
- Data Migration und Integration
- Anbindung der Offline-App
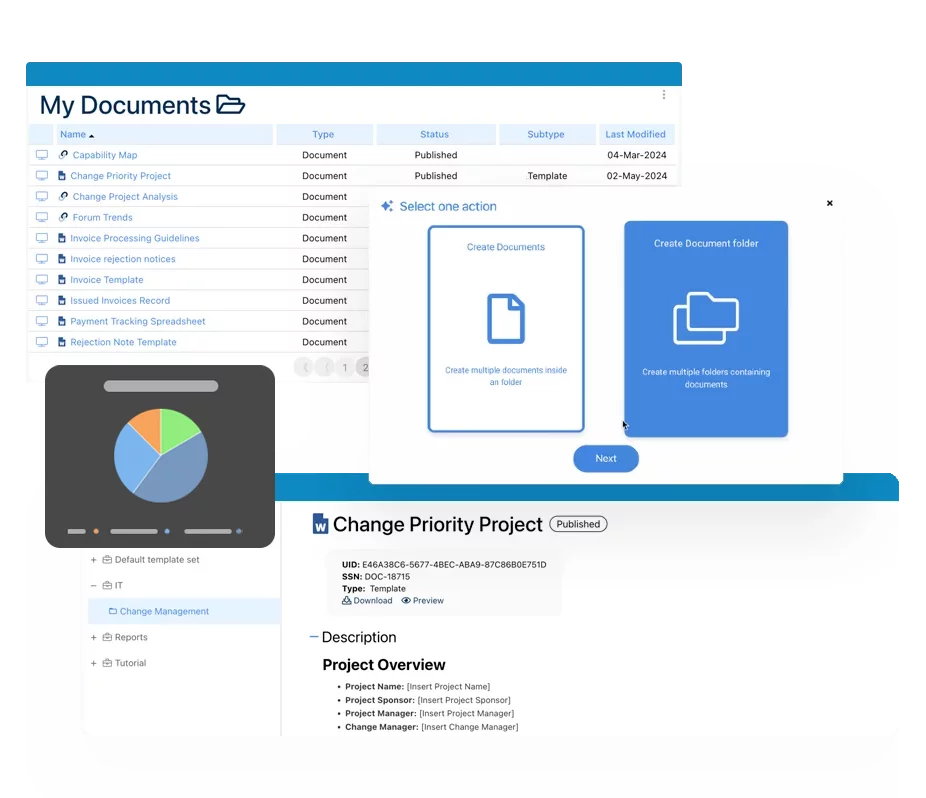
 Dokumenten-Management-System (DMS)
Dokumenten-Management-System (DMS)- Dokumentenlenkung Übersicht
- KI-Inhaltserstellung und -verbesserung
- Verwaltung von Richtlinien und Verfahren (SOP)
- Zusammenarbeit & Governance
- Data Migration und Integration
- Anbindung der Offline-App
 Elektronisches Qualitätsmanagementsystem (QMS)
Elektronisches Qualitätsmanagementsystem (QMS)- Überblick über das Qualitätsmanagementsystem
- Dokumente Control & Aufzeichnungen Mgmt
- Audit & Akkreditierung Mgmt
- Korrektur- und Präventivmaßnahmen
- Qualitätsereignis (nicht konform/konform), beschwerde, konformität
- Risikomanagement
- Management von Vorfällen
- Umwelt, Gesundheit und Sicherheit
- Produkt- und Lieferantenmanagement (SCAR)
- Ausbildung Management
- Control Management
- Aktionspunkte Management
- Management Review
- FMEA
- Pharmakovigilanz
- Data Migration und Integration
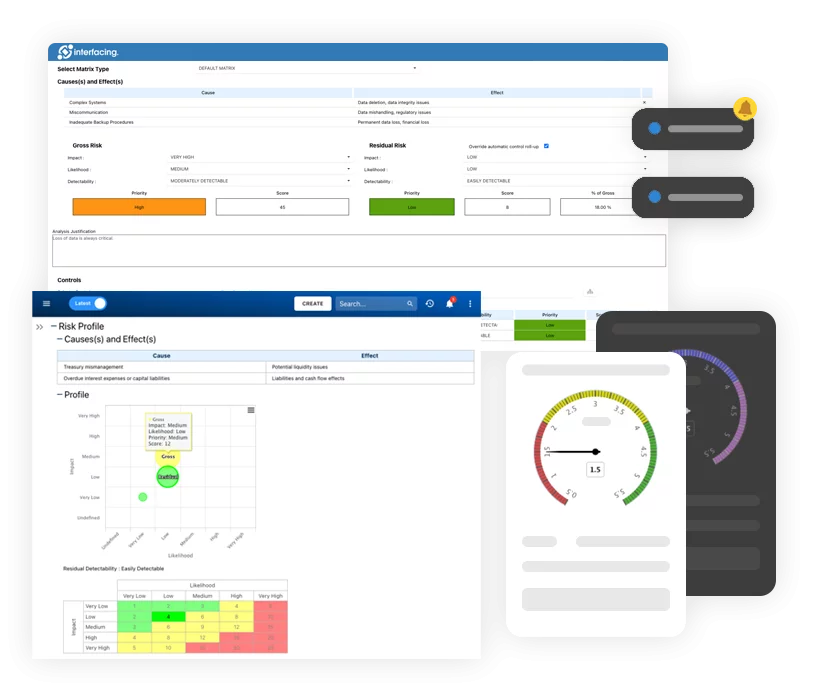
 Risk, Governance & Compliance (GRC)
Risk, Governance & Compliance (GRC)- Überblick zu Risiko, Governance & Compliance
- Risikomanagement und Kontrolle
- Einhaltung gesetzlicher Vorschriften
- Zusammenarbeit & Governance
- Data Migration und Integration
- Anbindung der Offline-App
 Schnelle Anwendungsentwicklung mit wenig Code (LC)
Schnelle Anwendungsentwicklung mit wenig Code (LC)- Low-Code-Automatisierungsplattform Übersicht
- Gestaltung elektronischer Webformulare (eFORMS)
- Low-Code-Automatisierungsplattform Übersicht
- Datenbank Tabelle Entity Designer
- Konzeption und Management von Aufgaben
- Entwurf und Verwaltung von BPMS-Anwendungen
- Integration mit externen Ressourcen und Anwendungen
- Definieren & Verwalten von Regeln/ Kontrollen/ Aktionen
- Elektronische Dienstleistungen
- Benutzer Startseite
- BAM (Business Activity Monitoring)
- Benutzerdefiniertes Dashboard-Design
- Data Migration und Integration
 Betriebliches Kontinuitätsmanagement (BCM)
Betriebliches Kontinuitätsmanagement (BCM)- BCM-Übersicht
- Analyse der geschäftlichen Auswirkungen
- Katastrophenschutz-Simulation
- Verwaltung der Handlungspunkte
- Verwaltung von Massenbenachrichtigungen
- Vermögensverwaltung
- Data Migration und Integration
 Unternehmensarchitektur (EA)
Unternehmensarchitektur (EA) Beratungsdienste
Interfacing ist hier, um Sie bei allen Transformationsinitiativen zu begleiten.
- Einhaltung gesetzlicher VorschriftenAnwendungsfälleLernzentrumRahmen & PraktikenEinhaltung gesetzlicher VorschriftenAnwendungsfälle
- Qualitätsmanagement-System (QMS)
- Digitale Transformation
- Kontinuierliche Verbesserung
- Governance, Risiko & Compliance
- Wissensmanagement
- Systemimplementierung (ERP, CRM…)
 Lernzentrum
Lernzentrum- Gemeinschaftsportal
- Webinare
- Videos
- Was ist BPM?
- BPMN 2.0-Symbolik
- Prozess-Mapping vs. Modellierung
- Glossar
 Rahmen & Praktiken
Rahmen & Praktiken - Über unsKundenerfolgPartnerÜber unsKundenerfolgPartner


Leading QMS Software for Pharmaceutical Companies


QMS Software for Pharmaceutical Companies
Please Select contact form.
AI-Powered Quality, Compliance & GxP Automation for Pharma

Unify processes, documentation, and compliance across the product lifecycle
Pharmaceutical organizations face constant pressure to maintain compliance with GxP, FDA 21 CFR Part 11, EU GMP Annex 11, and ICH Q10 while accelerating innovation and protecting patient safety.
Manual document control and disconnected systems can no longer keep up with the pace of regulatory change, global manufacturing, and digital transformation.
Interfacing’s AI-powered Quality Management System (QMS) helps pharmaceutical manufacturers unify processes, documentation, and compliance across the product lifecycle, from research and development to post-market vigilance.
The Challenge: Complexity in a Fast-Moving Digital World
In today’s hyper-connected economy, technology and media organizations must continuously adapt, integrating new tools, updating policies, and ensuring uninterrupted service delivery.
Manual governance processes and fragmented systems often lead to duplication, shadow IT, and non-compliance with frameworks like ISO 27001, GDPR, and SOC 2.
To maintain control at scale, enterprises need a single source of truth that aligns strategy, processes, and security controls, powered by AI for visibility and continuous improvement.


Why Leading Pharma Companies Choose Interfacing
Proven GxP Compliance: Digitally enforce 21 CFR Part 11, Annex 11, and ICH Q10 requirements.
End-to-End Traceability: Link every record, SOP, and CAPA to its related process and regulation.
AI-Powered Insight: Detect non-conformities, automate CAPA, and forecast recurring issues.
Digital Twin of the Organization (DTO): Visualize the full pharma value chain for smarter quality decisions.
Validated Environment: 21 CFR Part 11 / Annex 11–ready — audit trail, electronic signature, and version control.
Request a personalized demo of the AI-Integrated Management System (IMS) to see how global pharma leaders modernize their quality and compliance frameworks.
Streamline Your Pharmaceutical Quality Operations
With Interfacing’s integrated platform, you can:
Manage deviations, CAPA, and change control through automated workflows.
Digitize training management to ensure employee readiness and procedural adherence.
Simplify supplier qualification and audit readiness across your global network.
Ensure real-time visibility with compliance dashboards and KPIs.
Explore related resources:
→ FDA 21 CFR Part 820 Compliance
→ ISO 13485 Compliance Guide
→ GxP Risk Management & Compliance

How Interfacing Helps Pharmaceutical Organizations
Interfacing supports global pharma companies with:
Unified document, process, and risk management
AI-based process discovery for continuous improvement
Integrated quality event and CAPA lifecycle tracking
Secure, validated e-signature and access control
Multi-site, multi-language collaboration for regulated operations
Learn more about the AI-Integrated Management System or schedule a demo with our compliance experts.

Ensure Process & Quality Governance
Interfacing’s Enterprise Process Center® (EPC) allows you to define, document, and enforce ISO 9001 quality controls organization-wide. Policies, procedures, and SOPs are directly tied to processes and roles—ensuring your QMS is embedded in everyday operations with full transparency. .

Eliminate Manual Errors with AI-Driven QMS
Standardizing documentation and workflows within EPC removes the need for spreadsheets and disconnected systems. Our AI flags outdated procedures, suggests process improvements, and helps maintain version control—reducing the risk of non-compliance and audit issues.

Gain Full Audit Readiness & Traceability
EPC provides real-time traceability across all quality processes—from document approvals to CAPA workflows. With automated audit trails and visual process maps, you’re always ready for certification audits and internal reviews, no last-minute scrambling required

Improve Operational Efficiency Without Sacrificing Compliance
Interfacing’s QMS automates routine tasks such as change control, training sign-offs, and document updates. This not only frees up valuable staff time, but also ensures consistent adherence to ISO 9001 guidelines across all departments and geographies.

Reduce the Cost of Quality Compliance
With centralized process governance and AI-enhanced workflows, EPC reduces the overhead of maintaining your QMS. From smarter audit prep to fewer non-conformities, organizations save time, cut errors, and accelerate their path to ISO 9001 certification.

Build a Culture of Continuous Improvement
ISO 9001 is about more than passing audits—it’s about evolving your organization. Interfacing’s platform helps identify quality gaps, track corrective actions, and promote ongoing learning and accountability—turning compliance into a competitive advantage.
Warum Interfacing wählen?
Mit mehr als zwei Jahrzehnten Erfahrung in den Bereichen KI, Qualität, Prozesse und Compliance ist Interfacing nach wie vor ein führendes Unternehmen in der Branche. Bis heute hat das Unternehmen mehr als 500 erstklassige Unternehmen und Unternehmensberatungen aus allen Branchen und Sektoren bedient. Wir bieten weiterhin digitale, Cloud- und KI-Lösungen an, die es Organisationen ermöglichen, ihre Prozesse zu verbessern, zu kontrollieren und zu rationalisieren und gleichzeitig die Last der Einhaltung von Vorschriften und Qualitätsmanagementprogrammen zu verringern.
Wenn Sie weitere Informationen wünschen oder besprechen möchten, wie Interfacing Ihr Unternehmen unterstützen kann, füllen Sie bitte das folgende Formular aus.

Dokumentation: Transformation, Governance und Kontrolle vorantreiben
· Gewinnen Sie in Echtzeitd umfassende Einblicke in Ihre Abläufe.
· Verbessern Sie Governance, Effizienz und Compliance.
· Sorgen Sie für nahtlose Einhaltung von regulatorischen Standards.

eQMS: Automatisierung von Qualitäts- und Compliance-Workflows und Berichten
· Vereinfachen Sie das Qualitätsmanagement mit automatisierten Workflows und Überwachung.
· Optimieren Sie CAPA, Lieferantenaudits, Schulungen und verwandte Workflows.
· Verwandeln Sie Dokumentation in
umsetzbare Erkenntnisse für Quality 4.0.

Low-Code Rapid Application Development: Beschleunigung der digitalen Transformation
· Erstellen Sie benutzerdefinierte, skalierbare Anwendungen schnell.
· Reduzieren Sie Entwicklungszeit und -kosten.
· Passen Sie sich schneller an und bleiben Sie agil angesichts sich wandelnder Kunden- und Geschäftsanforderungen.

KI zur Transformation Ihres Unternehmens!
KI-gestützte Tools sind darauf ausgelegt, Abläufe zu optimieren, Compliance zu verbessern und nachhaltiges Wachstum voranzutreiben. Erfahren Sie, wie KI:
· Mitarbeiterfragen beantworten kann.
· Videos in Prozesse umwandelt.
· Empfehlungen zur Prozessverbesserung und zu regulatorischen Auswirkungen gibt.
· eForms, Prozesse, Risiken, Vorschriften, KPIs und vieles mehr generiert.
· Regulatorische Standards in fragmentierte Anforderungen zerlegt.
Request Free Demo
Document, analyze, improve, digitize and monitor your business processes, risks, regulatory requirements and performance indicators within Interfacing’s Digital Twin integrated management system the Enterprise Process Center®!
Kunden weltweit vertrauen auf uns
Mehr als 400 Unternehmen und Unternehmensberatungen von Weltrang



















































INTEGRATION
Kunden weltweit vertrauen auf uns
Mehr als 400 Unternehmen und Unternehmensberatungen von Weltrang